Serum/plasma miRNA marker associated with breast cancer and application thereof
A marker, breast cancer technology, applied in the field of genetic engineering and oncology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] The collection of embodiment 1 sample and the arrangement of sample data
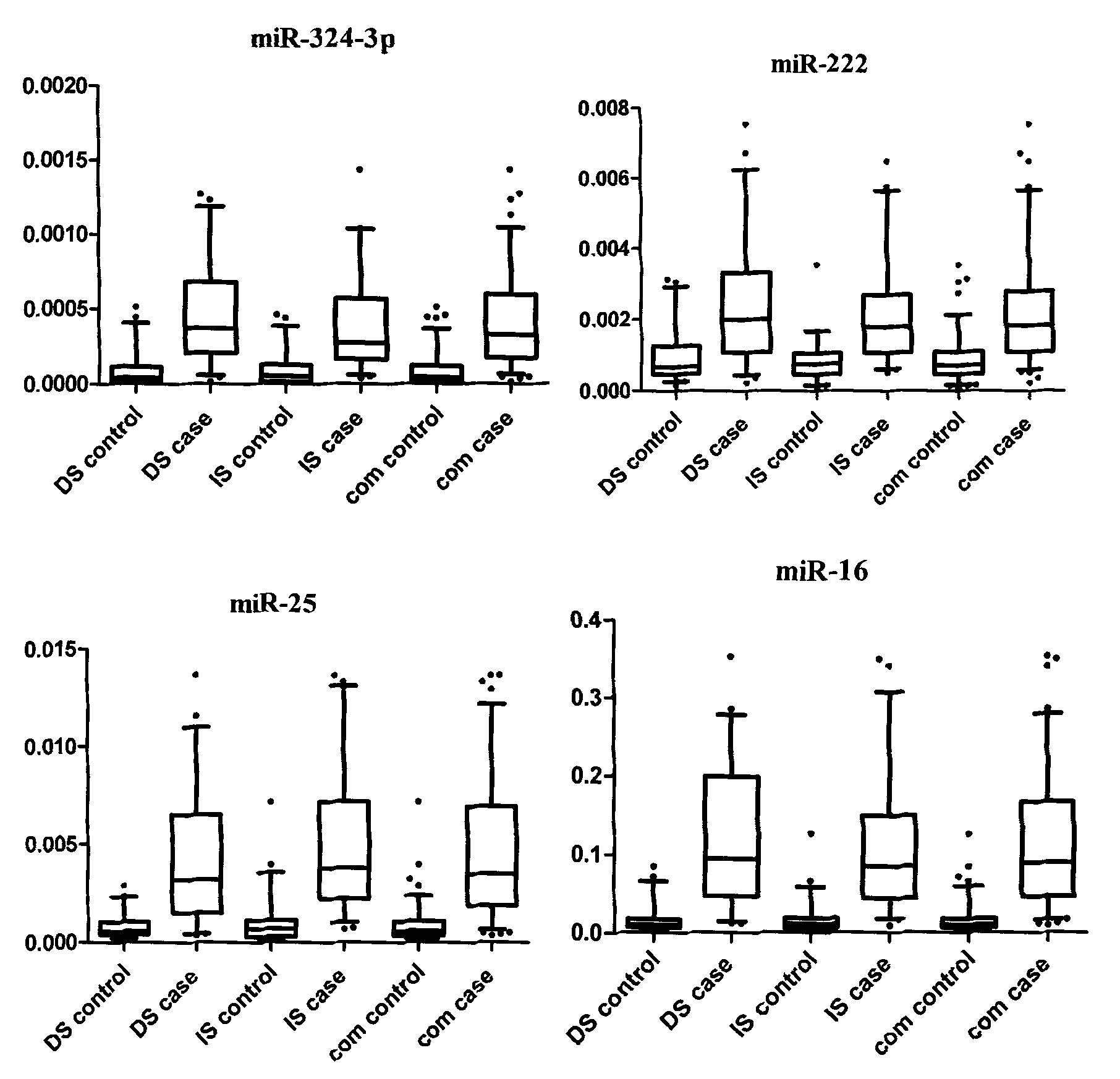
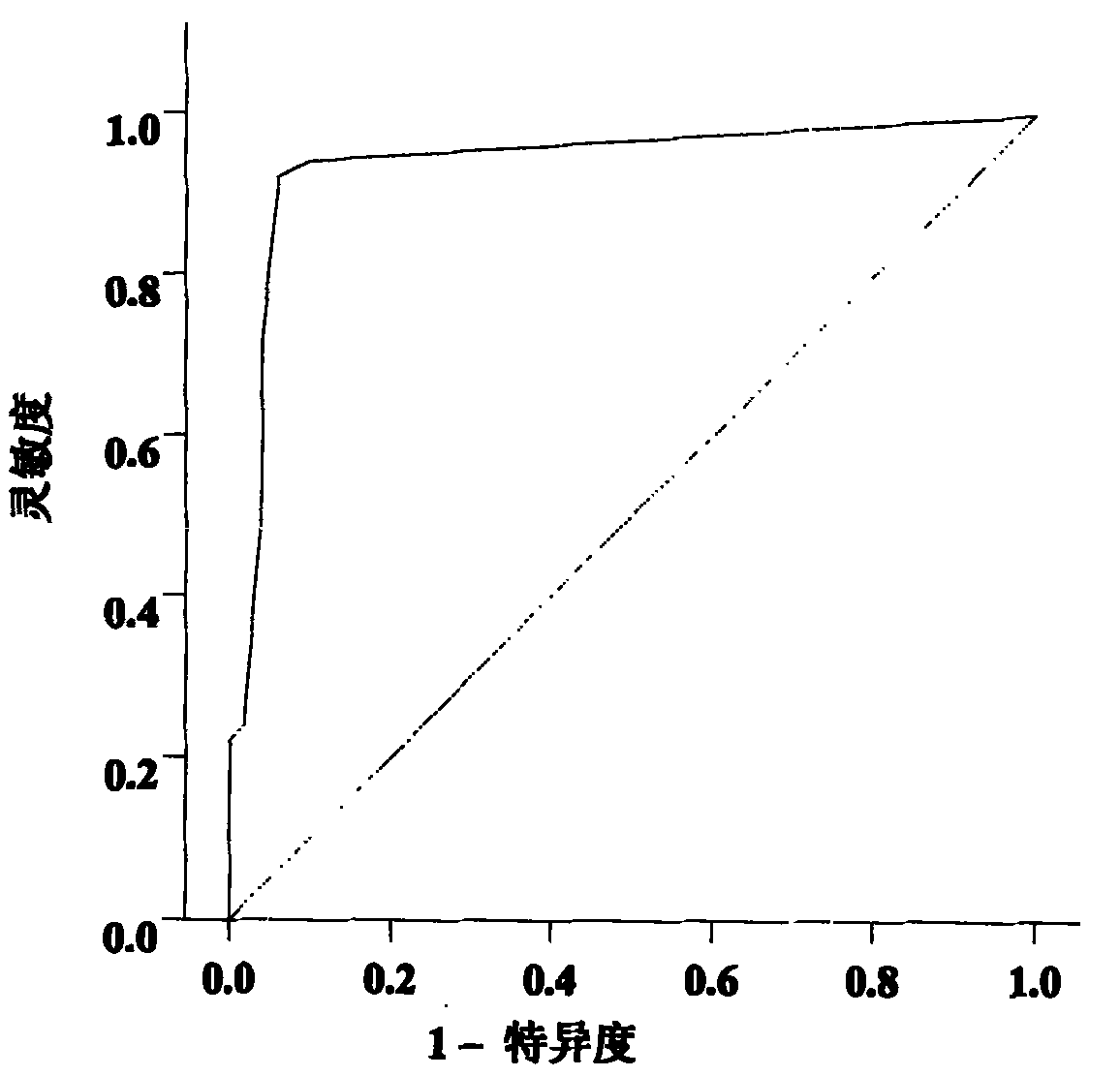
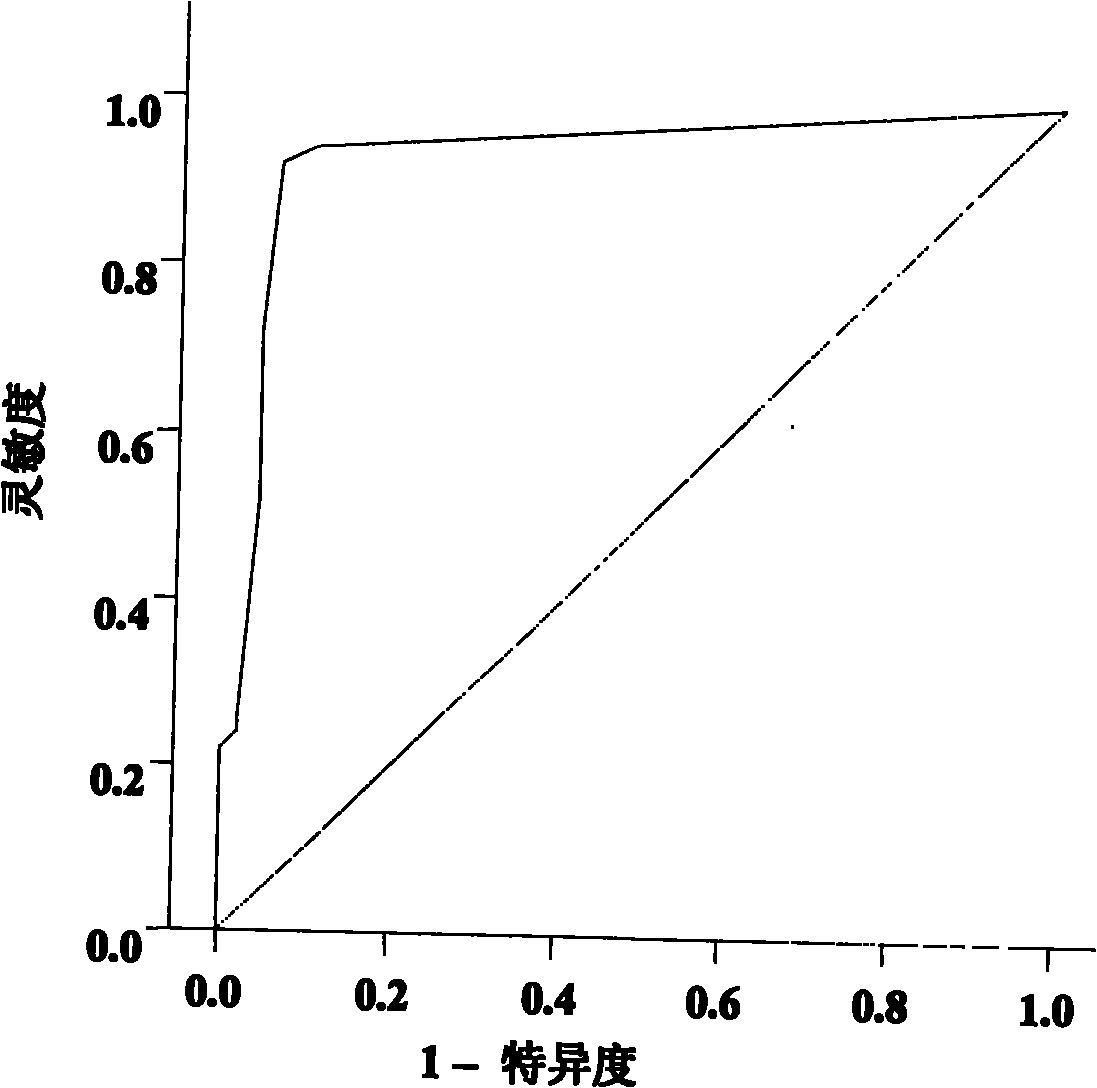
[0079] The inventor has collected a large amount of peripheral blood samples of breast cancer patients and controls from Jiangsu Cancer Hospital and Changzhou Wujin District since 2006 (the samples used for research are collected at the same period, and the conditions of sampling, sub-packaging and storage are uniform). For the sorting of sample data, the inventor selected 196 samples that met the following criteria as experimental samples for Solexa sequencing, TLDA chip detection and a series of subsequent qRT-PCR verifications:
[0080] 1. New breast cancer cases
[0081] 2. No surgery, radiotherapy and chemotherapy before blood collection, no preoperative radiotherapy and chemotherapy
[0082] 3. Healthy female controls matched with the age of the case
[0083] The demographic and clinical data of these samples were collected systematically.
Embodiment 2
[0084] Example 2 Solexa sequencing experiment of miRNA in serum / plasma
[0085] Among the 48 eligible breast cancer patients and 48 healthy female controls described above, the two groups were age-matched. The two groups of people were subjected to Solexa sequencing test to obtain relevant results. The specific steps are:
[0086] 1. Take 50ml of serum from patients in the "breast cancer case" group and the "healthy female control" group, and add an equal volume of Trizol reagent;
[0087] 2. Phase separation: place at room temperature for 15 minutes, then add chloroform according to the volume ratio of 0.2ml chloroform / 1ml Trizol reagent, shake for 15s, room temperature for 15 minutes, centrifuge at 12,000g, 4°C for 15 minutes;
[0088] 3. Transfer the aqueous phase to a new 50ml centrifuge tube, and remove the protein phase in 3 steps of phenol / chloroform;
[0089] 4. RNA precipitation: transfer the aqueous phase to a new centrifuge tube, add isopropanol according to the ...
Embodiment 3
[0097] TLDA chip detection of miRNA in embodiment 3 serum / plasma
[0098] The above 48 breast cancer patients and 48 healthy female controls subjected to Solexa sequencing were detected by TLDA chip to obtain relevant results. The specific steps are:
[0099] 1. Take 600 μl of serum from the "breast cancer case" group and the "healthy female control" group, and add 3 times the volume of Trizol reagent;
[0100] 2. Phase separation: place at room temperature for 15 minutes, add final concentration of 10 -4 Pmol / μl of cel-39 (TAKARA) was used as an internal reference, then chloroform equal to the volume of plasma was added, shaken for 50 s, room temperature for 15 min, 14,000 rpm, 4°C, and centrifuged for 15 min;
[0101] 3. RNA precipitation: transfer the water phase to a new 15ml centrifuge tube, add 1.5 times the volume of the water phase in absolute ethanol, and mix well;
[0102] 4. Enrich RNA with QIAGEN miRNeasy kit: pipette 700 μl of sample into the spin column each t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com