Method for producing porcine circovirus type II recombinant capsid protein subunit vaccine by utilizing silkworm bioreactor and products thereof
A porcine circovirus and subunit vaccine technology, applied in biochemical equipment and methods, botanical equipment and methods, viral peptides, etc., can solve the limitations of large-scale production and application of PCV2Cap protein, ineffective modification, foreign protein Low efficiency and other problems, to achieve the effect of enriching silkworm resources, high modification efficiency, and easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 Bombyx mori baculovirus expression system expresses recombinant porcine circovirus type 2 capsid protein
[0041] 1) Cloning the gene sequence ORF2 of porcine circovirus type 2 capsid protein, and connecting it with the baculovirus transfer vector to obtain the recombinant transfer vector;
[0042] The DNA of PCV2-SH strain (preservation number: CGMCC No. 2389) was extracted as a template for amplifying the Cap protein gene, and stored at -20°C for future use.
[0043] According to the complete PCV2 gene sequence in GenBank, a pair of primers were designed to amplify the PCV2 ORF2 open reading frame gene sequence. Add BamH I and Xhol I restriction sites (underlined) to the 5' ends of the upstream and downstream primers, respectively.
[0044] Upstream primer: 5′-CGC GGATCC ATGACGTATCCAAGGAGGC-3′;
[0045] Downstream primer: 5′-CCG CTCGAG GGGTTTAAGTGGGGGGTCT-3';
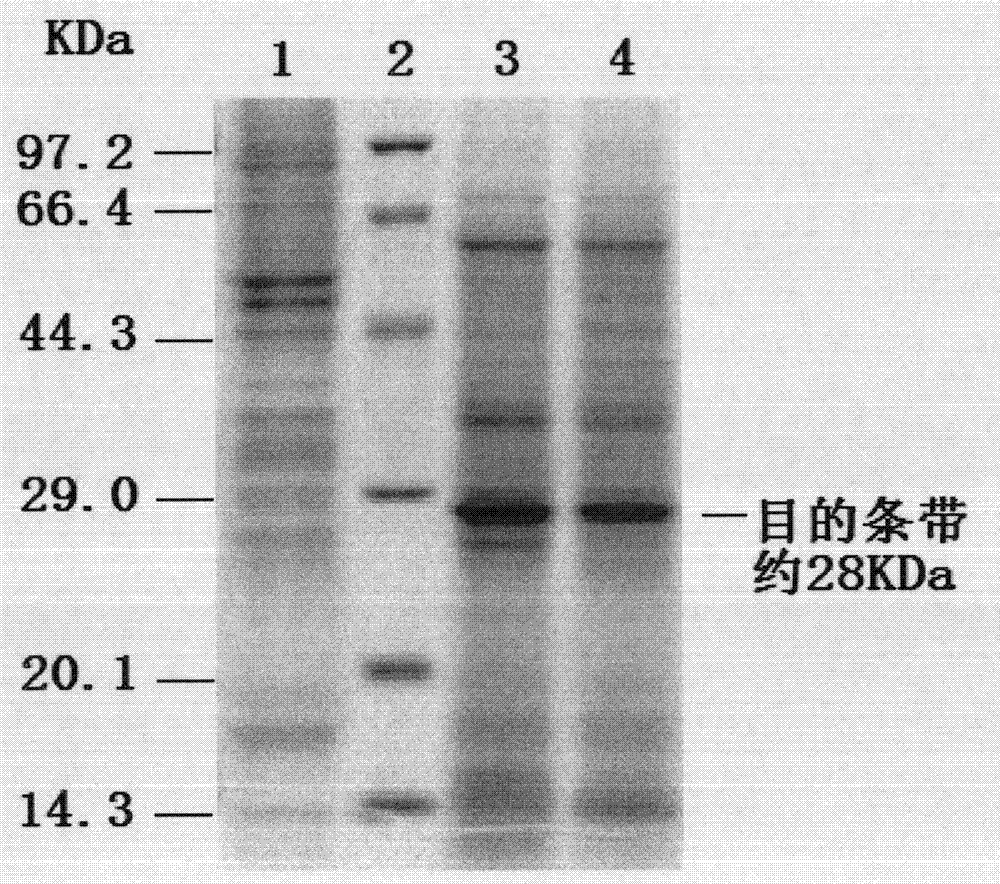
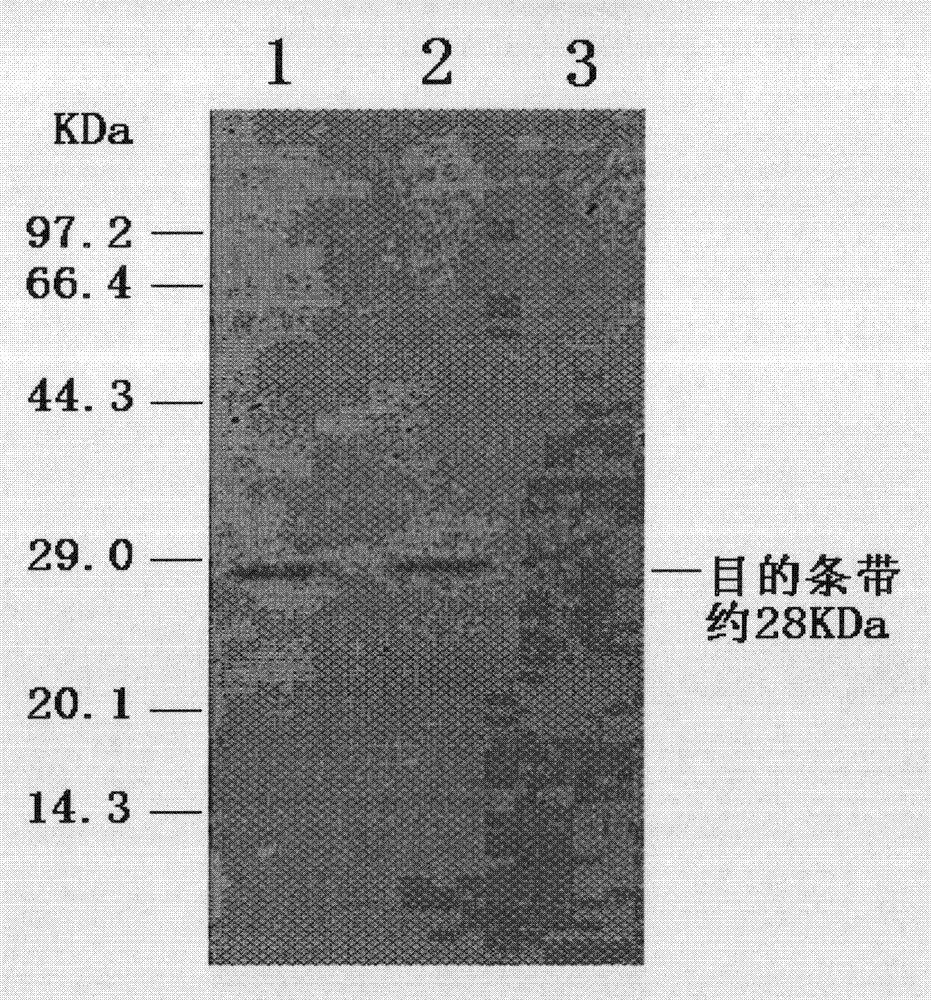
[0046]The PCV2 ORF2 gene fragment was amplified by PCR with the designed primers, and the b...
Embodiment 2
[0068] Efficacy detection of embodiment 2 recombinant PCV2 Cap protein subunit vaccine:
[0069] Screen pigs negative for major pathogens such as porcine circovirus, swine fever virus, porcine blue ear disease virus, and porcine parvovirus at the age of 10 to 14 days. Qualified piglets were randomly divided into 9 groups, 10 in each group, and 7 groups were given subunit vaccines containing different amounts of recombinant Cap protein (comprising 0.2 μg / head, 0.5 μg / head, and 1 μg of recombinant capsid protein respectively). / head portion, 2 μg / head portion, 5 μg / head portion, 10 μg / head portion and 15 μg / head portion), one group was used as a challenge control, and the other group was used as a negative control. Among them, group 1 was given a subunit vaccine containing 0.2 μg / head of recombinant Cap protein; group 2 was given a subunit vaccine containing 0.5 μg / head of recombinant Cap protein; group 3 was given 1 μg / head of recombinant Cap protein subunit vaccine; group 4 w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com