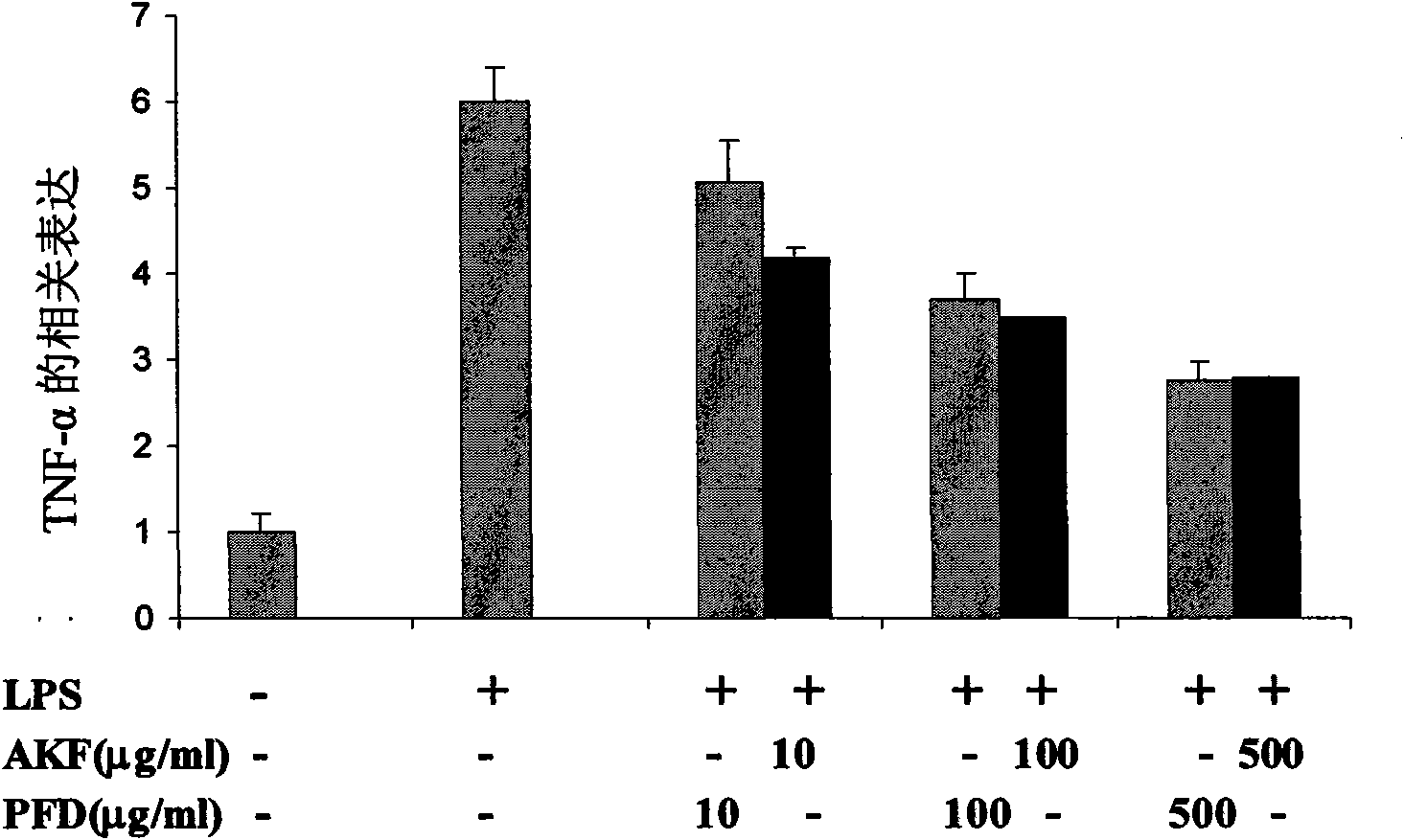Composition and method for treating proteinuria
A technology of high protein and composition, which is applied in the direction of drug combination, pharmaceutical formula, active ingredients of heterocyclic compounds, etc., and can solve problems such as reducing urinary protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
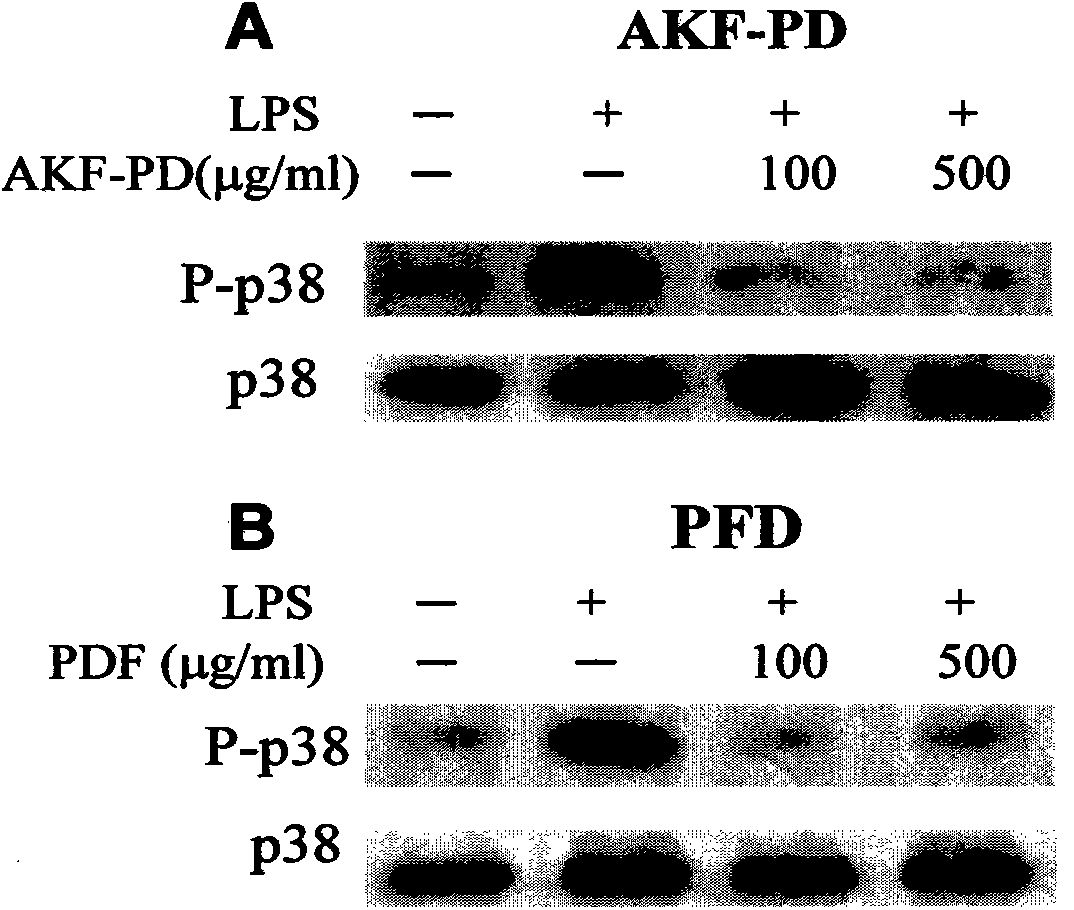
[0054] Example 1 Inhibition of activation of p38 by AKF-PD
[0055] There are three types of proteins in the MAPK family: Erk1 / 2, p38, and JNK. p38 kinase is activated by phosphorylation of the retained Thr-Gly-Tyr in its activation compartment. Activated p38 can further phosphorylate many targets in the cell and nucleus, leading to cellular responses such as apoptosis, inflammation and fibrosis. We first analyzed the anti-inflammatory effect of AKF-PD using the p38 delivery system in endotoxin-stimulated mouse macrophages (RAW264.7). Mouse macrophages (RAW264.7) were pretreated with AKF-PD or pirfenidone (PFD) for 4 hours, and then stimulated with endotoxin for 30 minutes. Such as figure 1 As shown, the p38 activity of endotoxin-treated cells was significantly increased. AKF-PD ( figure 1 A) and PFD ( figure 1 B) All of them significantly inhibited the activity of p38 stimulated by LPS. AKF-PD and PFD should have no effect on the stability of p38 protein, judging from ...
Embodiment 2
[0057] Inhibitory effects of AKF-PD and PFD on the synthesis of TNF-α mRNA in mouse macrophages
[0058] Macrophages are an important source of TNF-α and other pro-inflammatory cytokines for rheumatoid arthritis, and many pathways including p38MAPK may be involved in endotoxin-induced TNF-α production in these cells. Therefore we studied the effect of AKF-PD on the gene expression of TNF-α. The level of TNF-α mRNA in endotoxin-stimulated cells increased 6-fold. Both AKF-PD and PFD inhibited the synthesis of TNF-α mRNA in a dose-dependent manner ( figure 2 ). At about 500 μg / ml, the inhibition rate can reach 50%.
Embodiment 3
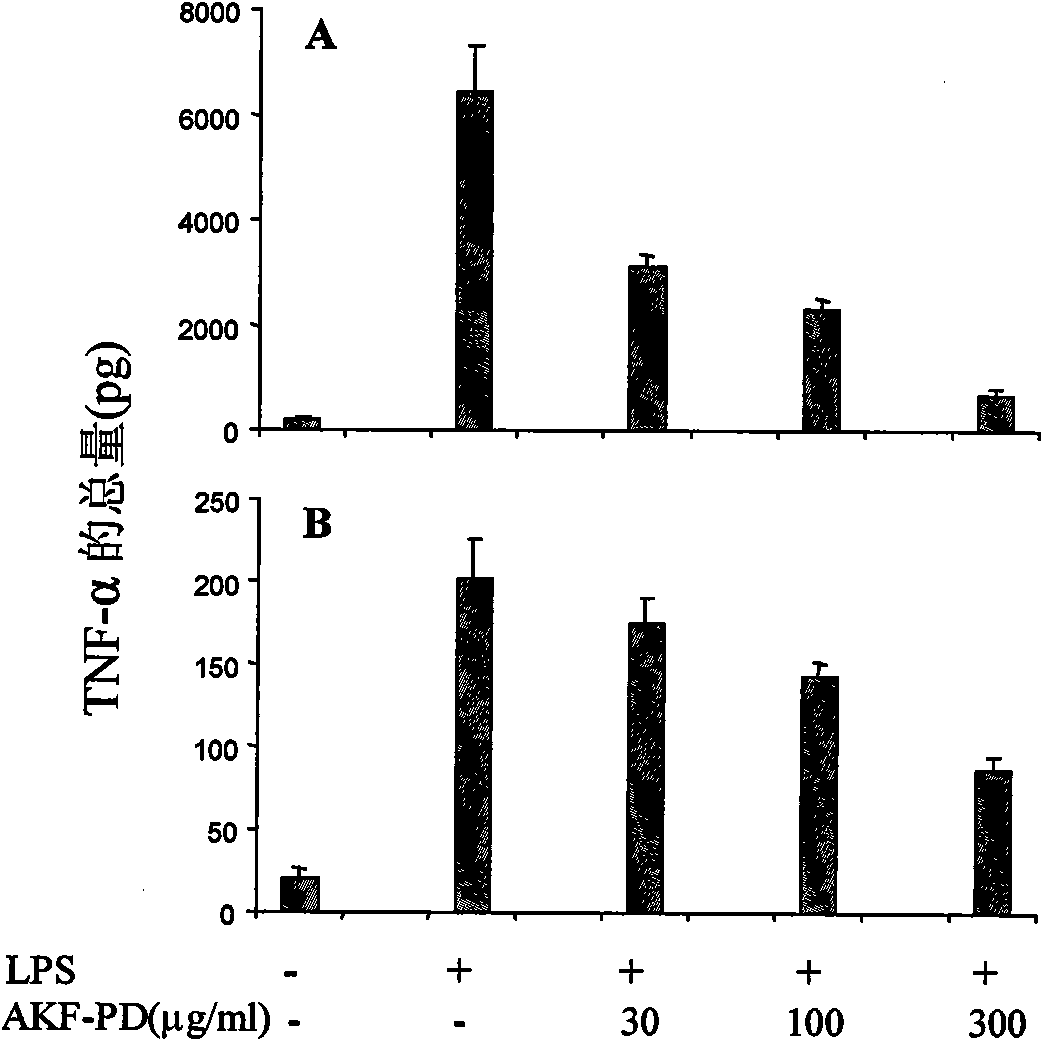
[0060] Inhibitory effect of AKF-PD on the synthesis and secretion of TNF-α protein in macrophages
[0061] The effects of AKF-PD on the synthesis and secretion of TNF-α protein were analyzed with endotoxin-activated mouse macrophages (RAW264.7). Cells were pretreated with AKF-PD and then activated with endotoxin. The secreted and intracellular TNF-α protein was measured simultaneously. Consistent with the effect of suppressing mRNA, cells pretreated with AKF-PD also inhibited the protein level of TNF-α in a dose-dependent manner ( image 3 ). AKF-PD at a concentration of 100 μg / ml can reduce the secretion of TNF-α by 64%. When the concentration increased to 300 μg / ml, AKF-PD could reduce the secretion of TNF-α by 89% ( image 3 A). AKF-PD can also reduce the amount of TNF-α in cells, but the inhibitory effect is very low, reaching about 50% inhibition at the concentration of 300 μg / ml ( image 3 B). Therefore, the above results indicate that AKF-PD can significantly and d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com