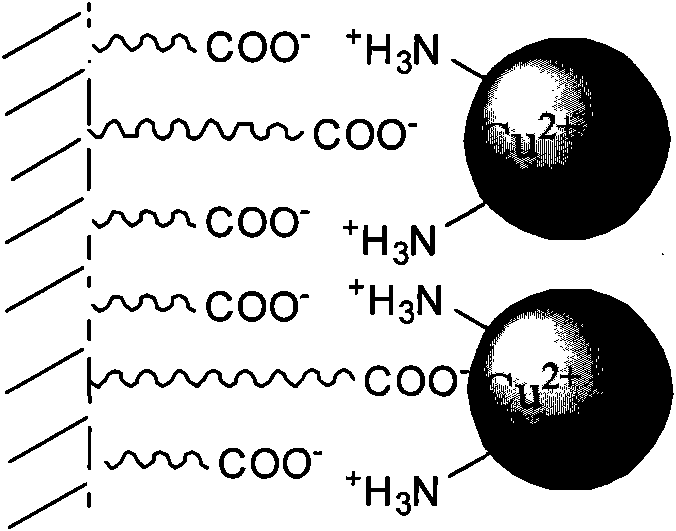Immobilized laccase, preparation method and application thereof
A technique for immobilizing laccase and laccase, applied in chemical instruments and methods, immobilized on/in organic carriers, wastewater treatment in processing, etc., can solve difficult industrial production and practical application, complicated processing technology, Solve problems such as low immobilization efficiency, achieve stable properties, simple processing technology, and firm adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The preparation of embodiment 1 iminocarboxylic acid type chelating resin immobilized laccase
[0021] (1) Soak 10g of commercially available iminocarboxylic acid type chelating resin (model LSC-100) in 500ml ethanol for 4h, after filtering off the ethanol, vacuum dry for 4h;
[0022] (2) Put the dried resin into 500ml of phosphate buffer solution containing 3000U laccase (pH=8.0), shake and adsorb at 4°C for 6-12h;
[0023] (3) Washing with a phosphate buffer solution of pH=8.0, and detecting the washing solution until no protein is detected in the washing solution.
[0024] (4) Measure the amount M1 of laccase added before fixation and the amount M2 of laccase remaining in the eluate after fixation by Coomassie brilliant blue staining method (Bradford method), and calculate the immobilization rate of laccase: the fixation rate of laccase Conversion rate=[(M1-M2) / M1]×100%. The immobilization rate of laccase was 92%.
Embodiment 2 3
[0025] Embodiment 2 Preparation of ternary carboxyl chloride resin immobilized laccase
[0026] (1) Soak 10g of commercially available ternary carboxyl-containing vinyl chloride resin (model C42) in 500ml of ethanol for 4h, filter off the ethanol, and vacuum-dry for 4h;
[0027] (2) Put the dried resin into 500ml of phosphate buffer containing 5000U laccase (pH=8.0), shake and adsorb at 4°C for 6-12h;
[0028] (3) Washing with a phosphate buffer solution of pH=8.0, and detecting the washing solution until no protein is detected in the washing solution.
[0029] (4) Measure the amount M1 of laccase added before fixation and the amount M2 of laccase remaining in the eluate after fixation by Coomassie brilliant blue staining method (Bradford method), and calculate the immobilization rate of laccase: the fixation rate of laccase Conversion rate=[(M1-M2) / M1]×100%. The immobilization rate of laccase was 86%.
Embodiment 3
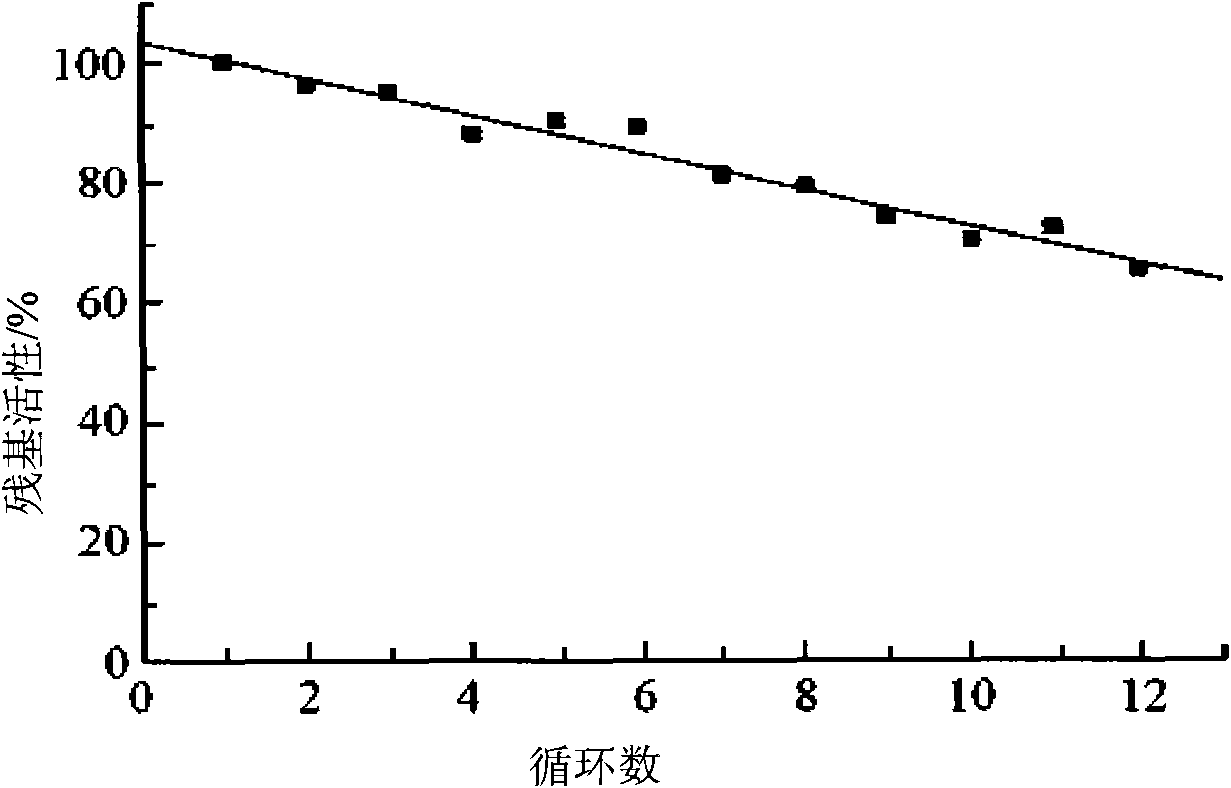
[0030] The thermostability of embodiment 3 immobilized laccase
[0031] The immobilized laccase and free laccase in Example 1 were respectively placed in a 70°C water bath for incubation, and samples were taken regularly to detect their guaiacol oxidation activity. The results showed that after the free enzyme was incubated in a 70°C water bath for 1 hour, the enzyme activity Significantly decreased, and the loss of enzyme activity reached more than 99% after 4 hours. However, the immobilized laccase only lost 19% of its enzyme activity when incubated for 4 hours under the same conditions, and still retained 56.5% of its enzyme activity after 8 hours, showing that the thermal stability of the enzyme was significantly improved after immobilization. The immobilized laccase of Example 2 is similar to this.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com