Derivative L of rhodamine B, preparation thereof and use thereof
A technology for derivatives and products, applied in the field of fluorescent probe detection, can solve the problems of complex synthesis and separation, high detection limit, large interference of coexisting ions, etc., and achieve the effects of simple synthesis steps, low detection limit, and easy purification.
Inactive Publication Date: 2010-12-15
YANTAI DONGRUN INSTR
View PDF1 Cites 25 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Among the reported fluorescent molecular probes, most of them have the following disadvantages: complex synthesis and separation, large interference of other coexisting ions in the test process, high detection limit, and response signals of fluorescent quenching probes, etc., which are harmful to metal ions The detection has brought great impact and inconvenience
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
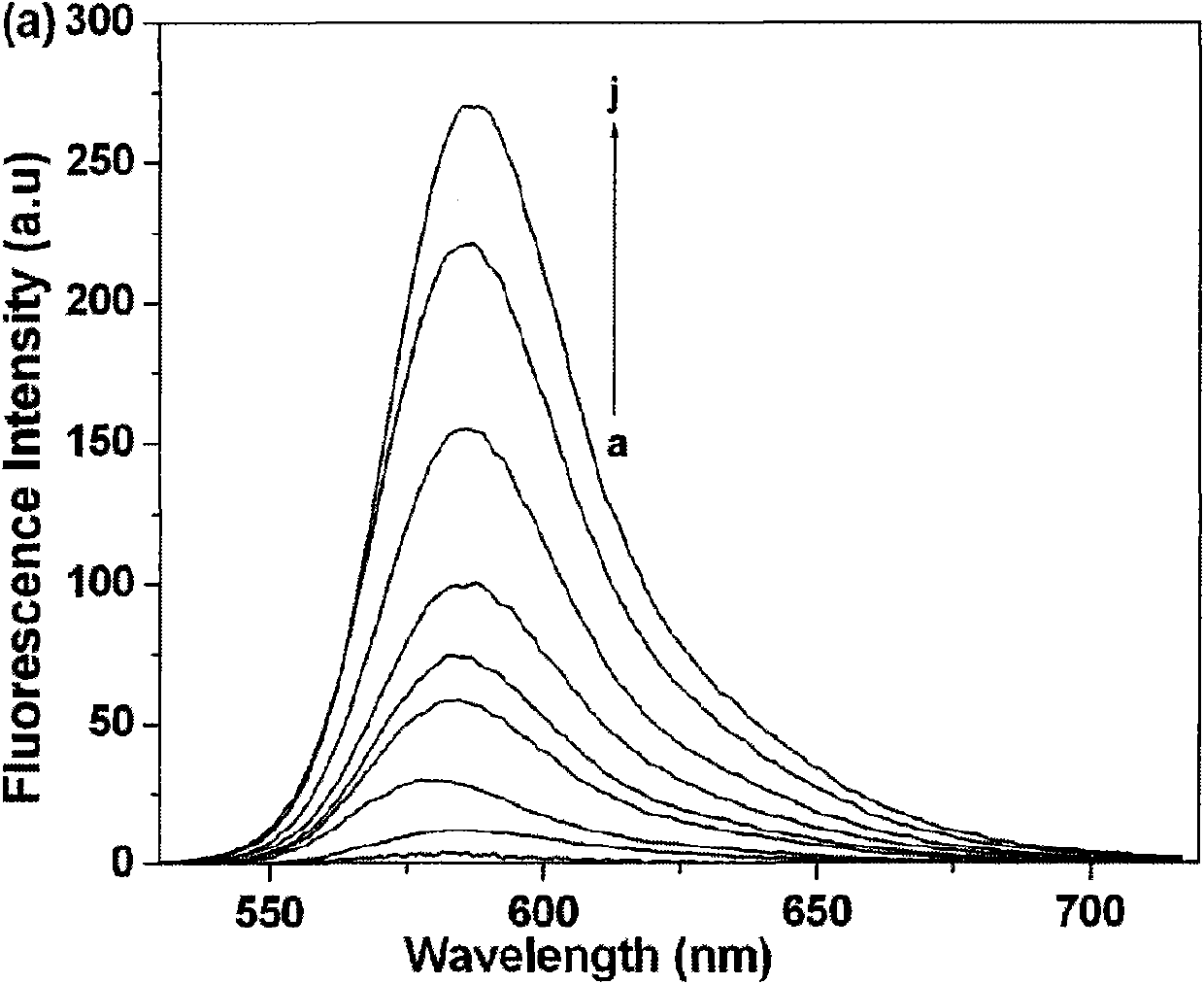
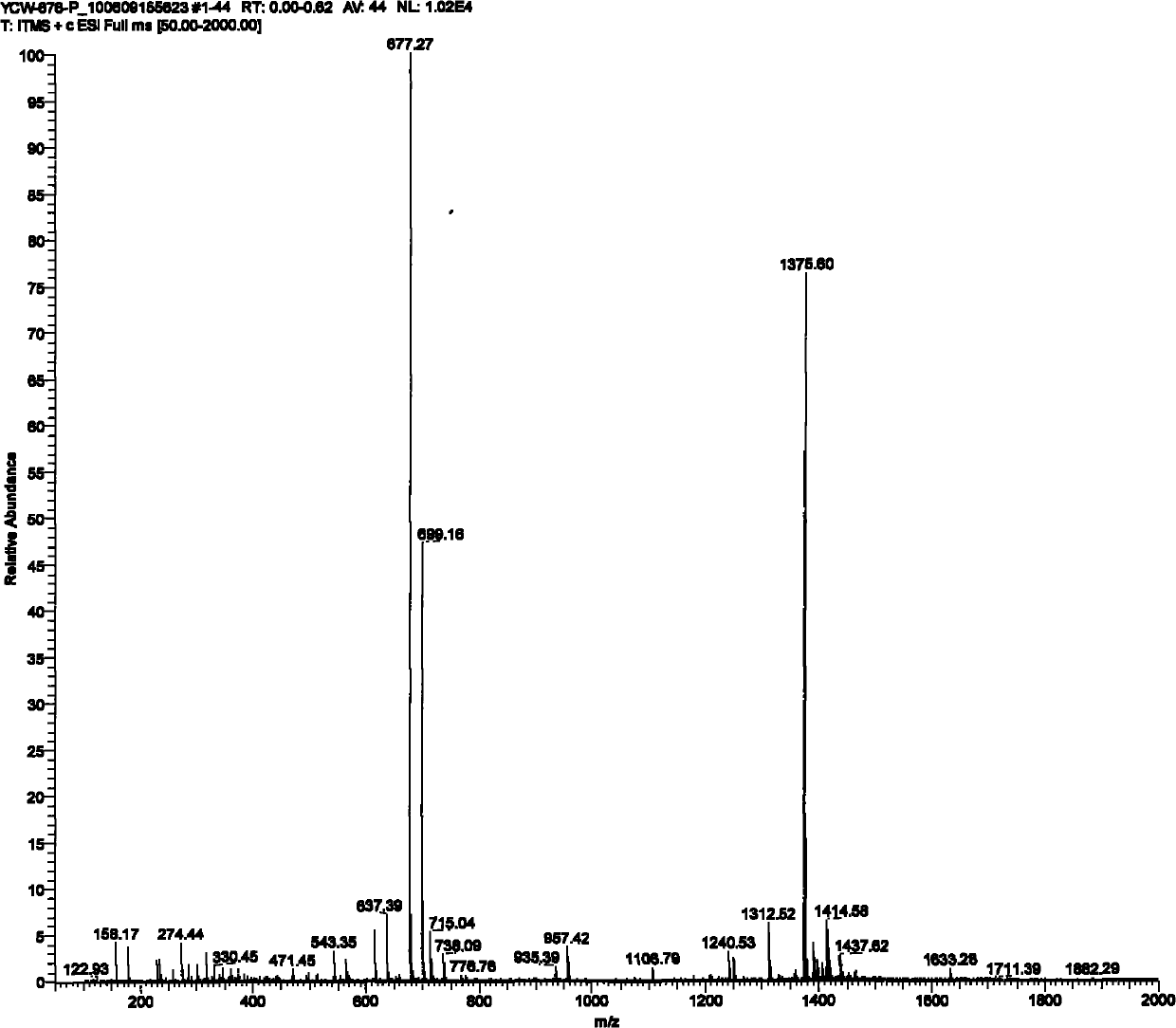
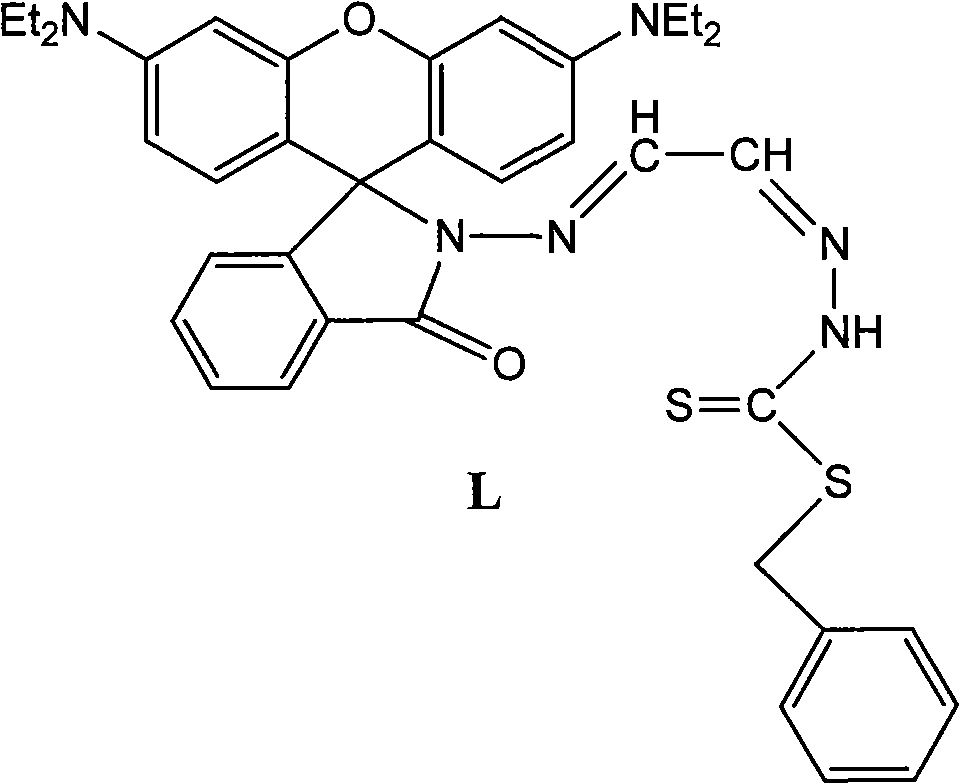
The invention relates to fluorescent probe detection, in particular to a derivative L of rhodamine B, preparation thereof and use thereof. The derivative L of the rhodamine B has a structural formula (I). The preparation method of the derivative L of the rhodamine B comprises: firstly, reacting the product of the hydrazinolysis of the rhodamine B with glyoxal to obtain an intermediate compound; and secondly, reacting the intermediate compound with benzyldithiocarbazate in a molar ratio of 1:1 in absolute ethanol under a refluxing condition for 4 to 6 hours, cooling, filtering precipitated solid, washing with absolute ethanol and ethyl ether in turn and drying under vacuum to obtain a target compound. The derivative L of rhodamine B can serve as a fluorescent probe. In the invention, the derivative L of rhodamine B is obtained by an effective synthesis means, the copper ion selectivity is high, and the detection of copper ions can be realized on the basis of optimized experimental conditions.
Description
technical field The invention relates to fluorescent probe detection, in particular to a rhodamine B derivative and its preparation and application. Background technique Rhodamine B derivatives have excellent optical properties and are widely used in metal ion fluorescent probes. Fluorescent probes based on rhodamine B derivatives have been applied to the detection of mercury ions, lead ions, iron ions, chromium ions, and copper ions. Among the reported fluorescent molecular probes, most of them have the following disadvantages: complex synthesis and separation, large interference of other coexisting ions in the test process, high detection limit, and response signals of fluorescent quenching probes, etc., which are harmful to metal ions Detection has brought great influence and inconvenience. Contents of the invention The object of the present invention is to provide a rhodamine B derivative and its preparation and application. To achieve the above object, the techn...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07D491/107C09K11/06G01N21/64
Inventor 陈令新于春伟张军王锐
Owner YANTAI DONGRUN INSTR
Features
- Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com