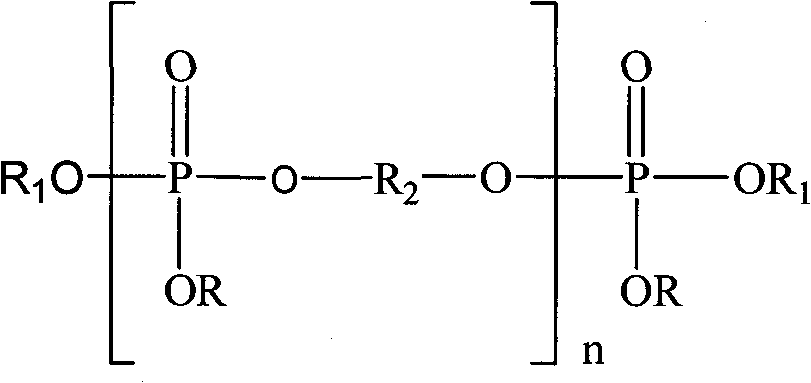
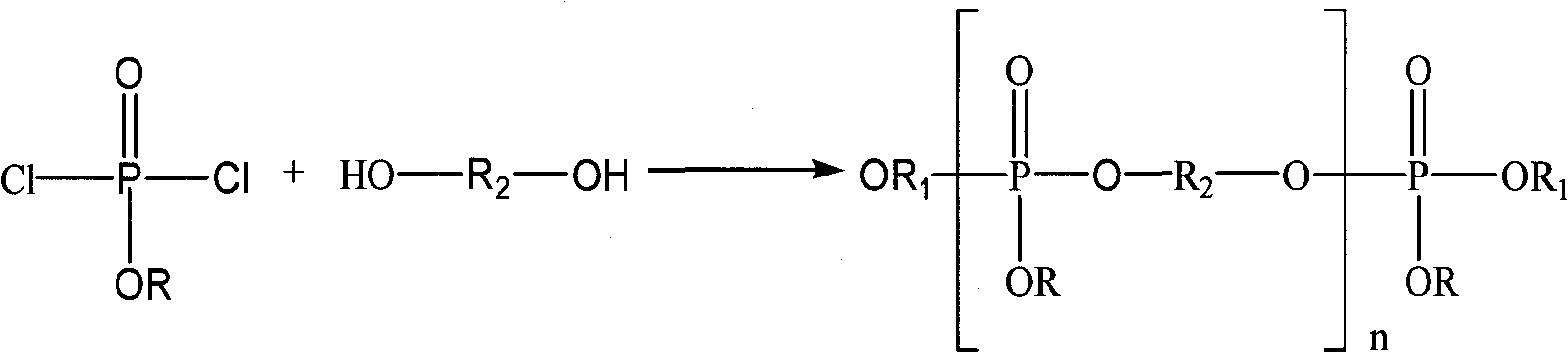
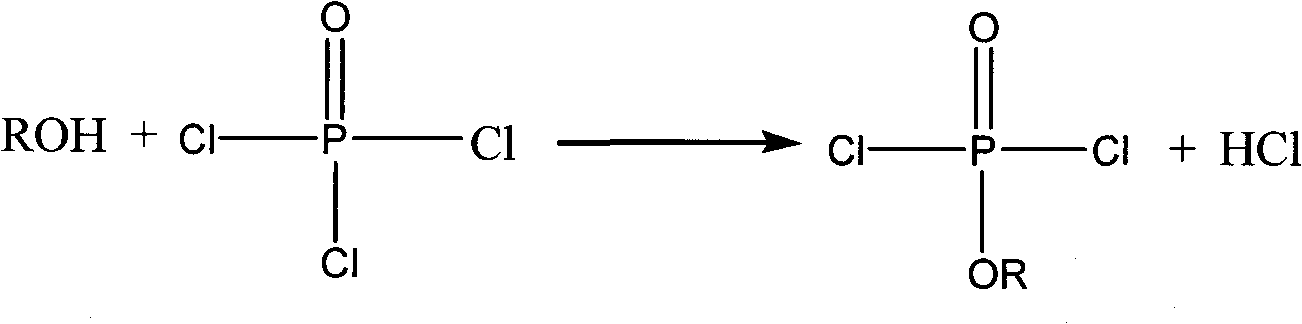Preparation method of oligomerization phosphate polyalcohol
A technology of oligomeric phosphate and polyol, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve the problems of reducing the aging time of flame retardancy, poor safety, and the risk of skin burns, etc. problem, to achieve the effect of durable flame retardant performance and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1, 1 mol and 153.5 g of phosphorus oxychloride were dissolved in 200 mL of dichloromethane, and then added to a four-necked flask equipped with stirring, thermometer, reflux condenser and liquid dropping device. Start stirring, slowly add 1 mol of methanol with a mass of 32g, cool down in a water bath during the dropwise addition, and control the reaction temperature at 30±5°C, absorb the hydrogen chloride gas generated during the dropwise addition with water, after the dropwise addition, keep warm and reflux for 4h until No hydrogen chloride gas escaped, and a reaction solution containing monoalkyl dichlorophosphate was obtained. Then add 2 mol of acid-binding agent triethylamine with a mass of 202 g and 0.31 g of catalyst aluminum trichloride to the reaction solution, control the reaction temperature at 20±5°C, slowly add 1.1 mol of ethylene glycol with a mass of 68.3 g After the addition, reflux for 10 hours until no hydrogen chloride gas escapes to obtain a ...
Embodiment 2
[0038] Example 2, 1 mol of phosphorus oxychloride with a mass of 153.5 g was dissolved in 200 mL of dichloromethane, and then added to a four-necked flask equipped with stirring, thermometer, reflux condenser and liquid dropping device. Start stirring, slowly add 1 mol of absolute ethanol with a mass of 46g, cool down in a water bath during the dropwise addition, and control the reaction temperature at 30±5°C, absorb the hydrogen chloride gas generated during the dropwise addition with water, and keep warm and reflux for 4 hours after the dropwise addition , until no hydrogen chloride gas escapes to obtain a reaction solution containing monoalkyl dichlorophosphate. Add 2 mol and 202 g of acid-binding agent triethylamine and 0.23 g of catalyst aluminum trichloride to the reaction solution, control the reaction temperature at 20±5° C., slowly add 1.5 mol of ethylene glycol with a quality of 93.1 g, add After completion of the reflux reaction for 16 hours, until no hydrogen chlor...
Embodiment 3
[0039] Example 3, 1 mol and 153.5 g of phosphorus oxychloride were dissolved in 200 mL of dichloromethane, and then added to a four-necked flask equipped with stirring, thermometer, reflux condenser and liquid dropping device. Start stirring, slowly add 1 mol of n-propanol with a mass of 60.1g, cool down in a water bath during the dropwise addition, and control the reaction temperature at 30±5°C, absorb the hydrogen chloride gas generated during the dropwise addition with water, after the dropwise addition, keep warm and reflux 5h, until no hydrogen chloride gas escapes, the reaction solution containing monoalkyl dichlorophosphate is obtained. In the reaction solution, add 2mol, a quality of 202g acid-binding agent triethylamine and 0.28g catalyst aluminum trichloride, control the reaction temperature 20±5°C, slowly add 1.9mol of ethylene glycol with a mass of 117.9g, and then reflux for 20h until no hydrogen chloride gas escapes to obtain oligomeric phosphate polyol, triethyla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com