Prasugrel hydrobromide polymorph and preparation method thereof
A technology of prasugrel hydrobromide and crystal form, which is applied in the field of synthesis of antithrombotic drug prasugrel hydrobromide and its polymorphs, and can solve the problem of prasugrel hydrobromide crystal form not seen Issues such as reporting, not providing data support with good stability, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
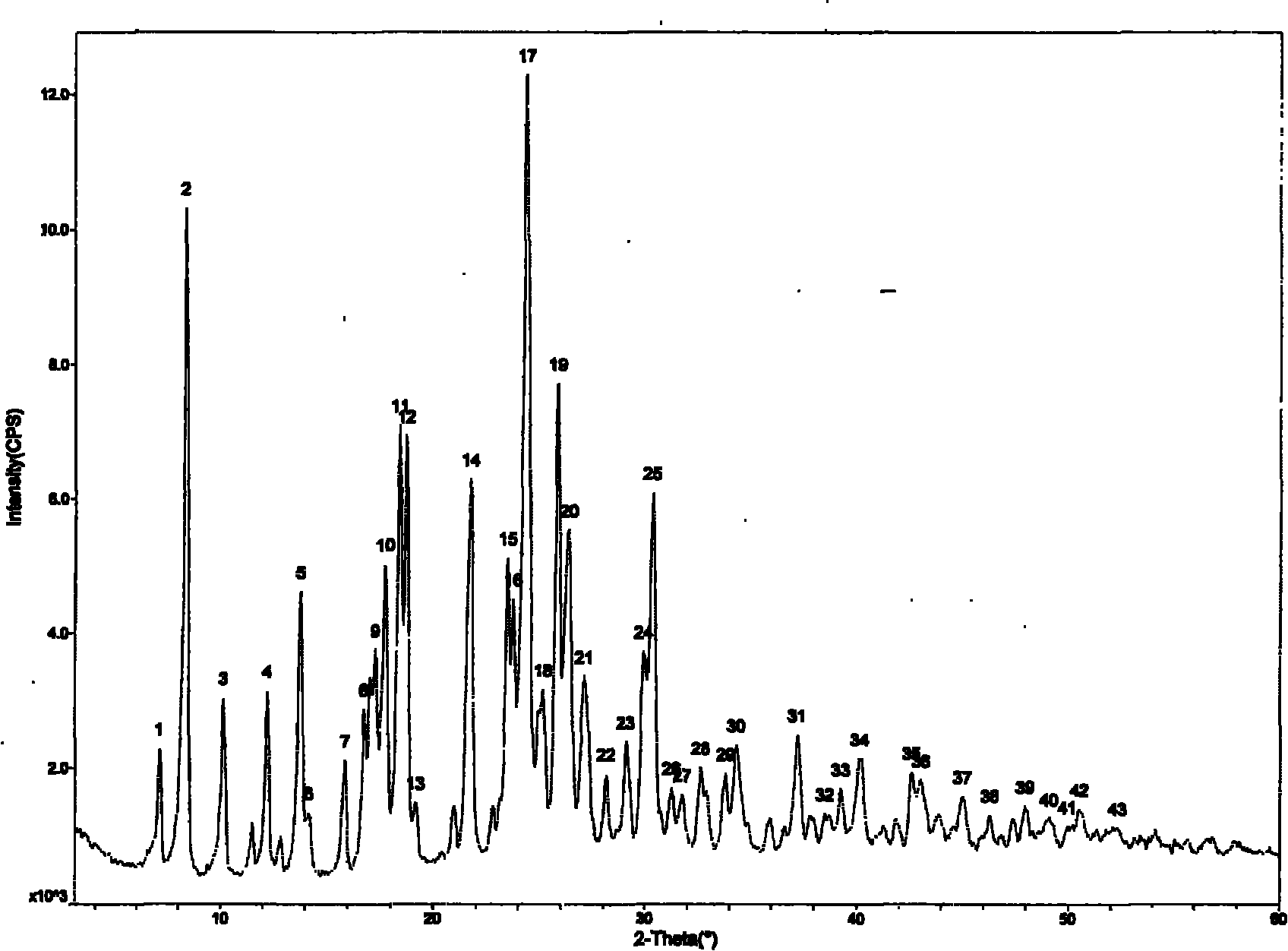
[0045] Example 1: Preparation of Form A of prasugrel hydrobromide.
[0046] Dissolve 10 g of prasugrel base in 100 ml of acetone, add dropwise to the organic solution 50 ml of acetone solution dissolved with 1 times the equivalent of hydrobromic acid, as the dropwise addition proceeds, solids slowly precipitate out, and react at room temperature for 1 hour , and suction filtered to obtain 8 g of white solid, with a yield of 85%. Melting point: 133-135°C. As determined by X-ray powder diffraction, it was shown that the generated crystal form was Form A.
Embodiment 2
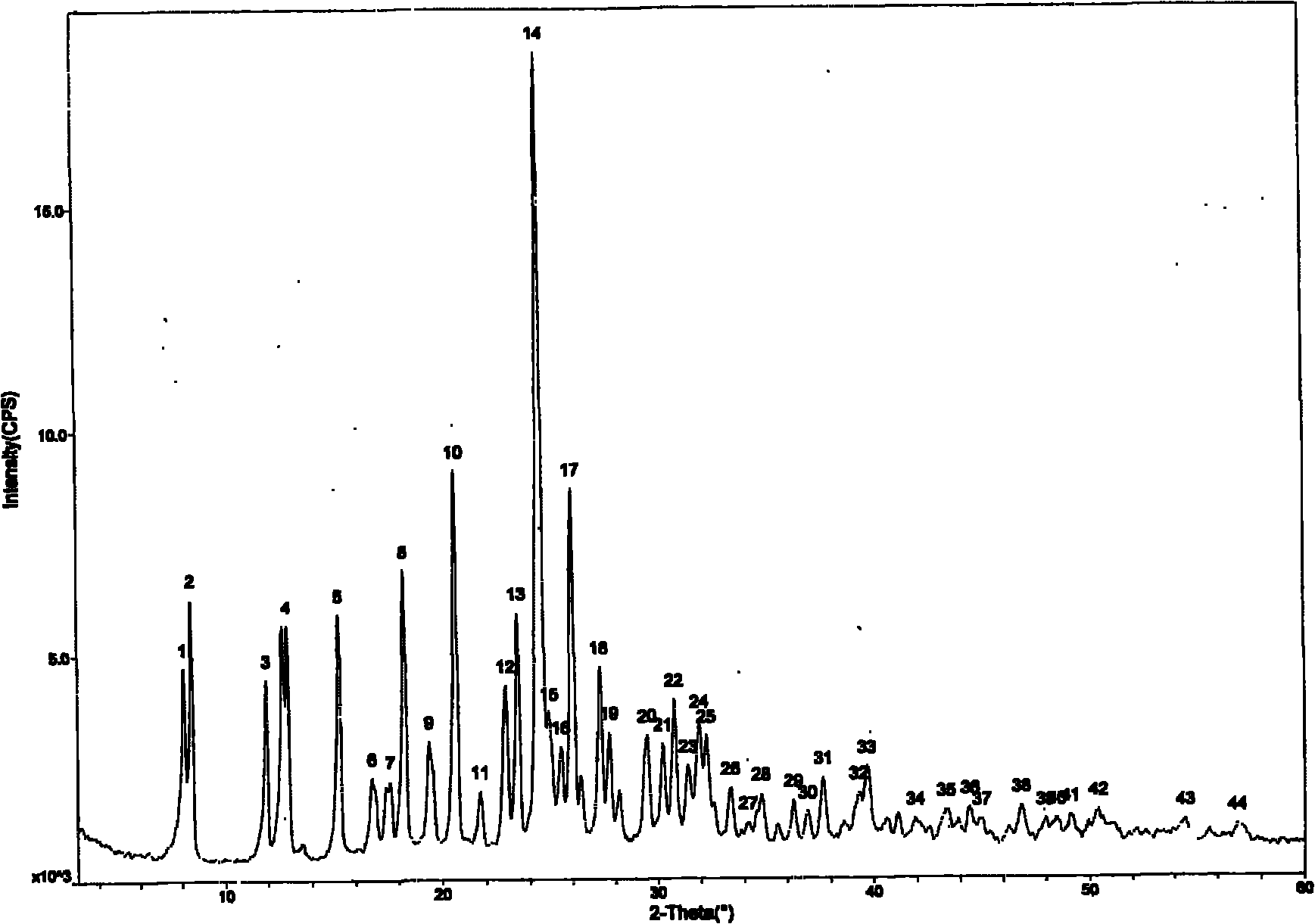
[0047] Example 2: Preparation of prasugrel hydrobromide crystal form B.
[0048] Take 10g of prasugrel hydrobromide crystal form A, add 50ml of ethanol, heat and stir until dissolved, reflux for half an hour, stop heating, cool naturally, stir and crystallize for 3h, filter with suction to obtain a white solid, dry in vacuum at 40° for 3h, and get Crystalline powder, melting point: 138-140°C. As determined by X-ray powder diffraction, it was shown that the generated crystal form was Form B.
Embodiment 3
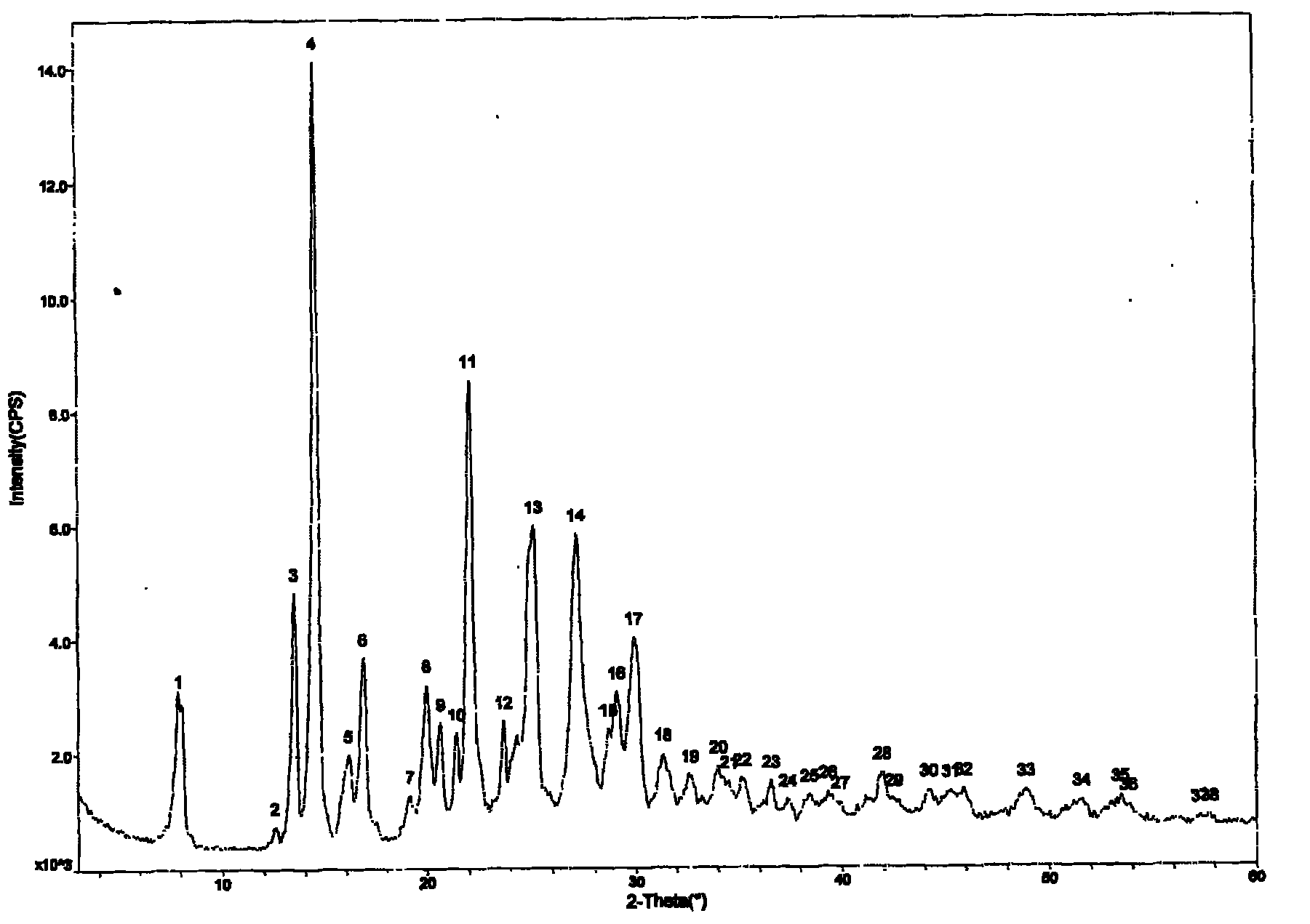
[0049] Example 3: Preparation of Prasugrel Hydrobromide Form C.
[0050] Take 10g of prasugrel hydrobromide crystal form A, add 150ml of ethyl acetate, heat to reflux for 1 hour, suction filter while it is hot to obtain a white solid, dry it in vacuum at 40° for 3 hours, and obtain a crystalline powder, melting point: 163-165°C . As determined by X-ray powder diffraction, it was shown that the generated crystalline form was crystalline form C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com