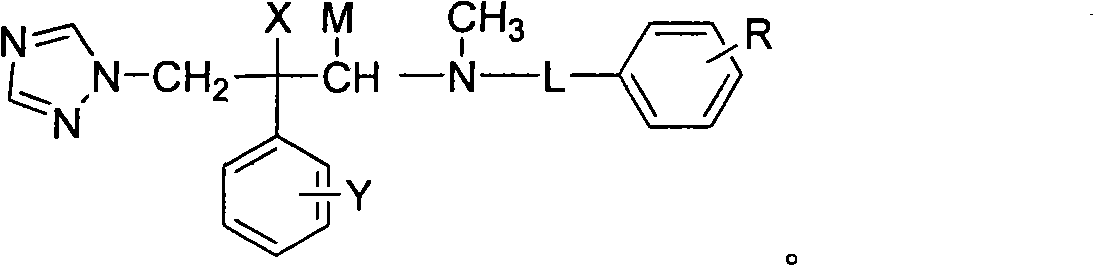
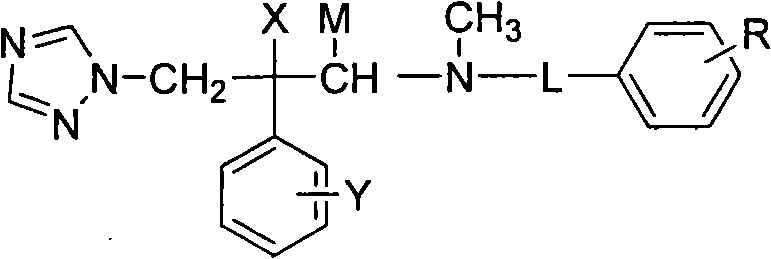
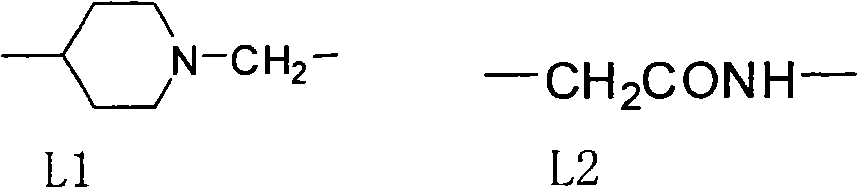Nitrogen methyl side chain-substituted triadimenol antifungal compound and preparation method thereof
A compound, the technology of triazole alcohol, applied in the field of medicine, can solve the problems such as the unseen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Embodiment 1: the preparation of 2-chloro-2', 4'-difluoroacetophenone (II)
[0107] 100g (0.747mol) of anhydrous aluminum chloride and 75.33g (0.667mol) of m-difluorobenzene (I) were placed in a 500mL three-necked flask, stirred at room temperature, and 75.33g (0.667mol) of chloroacetyl chloride was slowly added dropwise After the dropwise addition, continue to stir at room temperature for 30 minutes, slowly raise the temperature to 50°C, continue to stir at this temperature for 5 hours, pour the reaction solution into ice water, precipitate crystals, and filter to obtain a solid. Extract twice, combine the dichloromethane extracts, wash with water until neutral, dry over anhydrous sodium sulfate, filter, recover the solvent to obtain a solid, combine the solid obtained twice and recrystallize with methanol to obtain 2-chloro-2',4' -Difluoroacetophenone: 107.38g, yield 87.2%, melting point: 46-47°C.
Embodiment 2
[0108] Example 2: Preparation of 2-(1H-1,2,4-triazol-1-yl)-2',4'-difluoroacetophenone (III)
[0109] Triazole 27.2g (0.4mol), TEBA0.4g, anhydrous K 2 CO 3 41.56g (0.3mol) was added to 180mL of CH 2 Cl 2 Suspension was obtained; 2-chloro-2', 4'-difluoroacetophenone (II) 38.2g (0.2mol) was dissolved in 60mL CH 2 Cl 2 Add it dropwise to the above-mentioned 180mL suspension in an ice bath, and the drop is completed in about 1.5 hours. After the drop is completed, react at 0-5°C for 5 hours, and react at room temperature for 24 hours. Then filter, filter cake with CH 2 Cl 2Wash several times, collect the filtrate, wash the filtrate 3 times with water, each 100mL, anhydrous Na 2 SO4 dried, filtered, distilled CH 2 Cl 2 , dissolve the residue in 100 mL of anhydrous ethyl acetate, stir and add concentrated nitric acid dropwise until the yellow solid no longer precipitates, filter, wash the filter cake several times with a small amount of ethyl acetate, dry it, dissolve it in...
Embodiment 3
[0110] Example 3: Preparation of 1-[2-(2,4-difluorophenyl)-2,3-epoxypropyl]-1H-1,2,4-triazole mesylate (IV)
[0111] Take 2-(1H-1,2,4-triazol-1-yl)-2',4'-difluoroacetophenone (III) 29.8g (0.115mol), trimethyl iodine oxysulfide 25.3g ( 0.115mol), 1.6g of trimethylhexadecylammonium bromide, put into a 500mL three-necked flask, add 180mL of toluene and 225mL of 20% sodium hydroxide solution (w / w), heat at 60°C for 3 hours, and the reaction is completed Finally, separate the toluene layer, extract the water layer with toluene (100mL×2), combine the toluene layers, wash with water until neutral, and recover most of the toluene, dilute the residual solution with 120mL ethyl acetate, and add the solution dropwise at 0°C 8.3 g of ethyl acetate of methanesulfonic acid (2 mL), precipitated a pale yellow solid, filtered, and recrystallized from methanol to obtain compound IV: 21.71 g, yield 56.7%, melting point: 128-129°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com