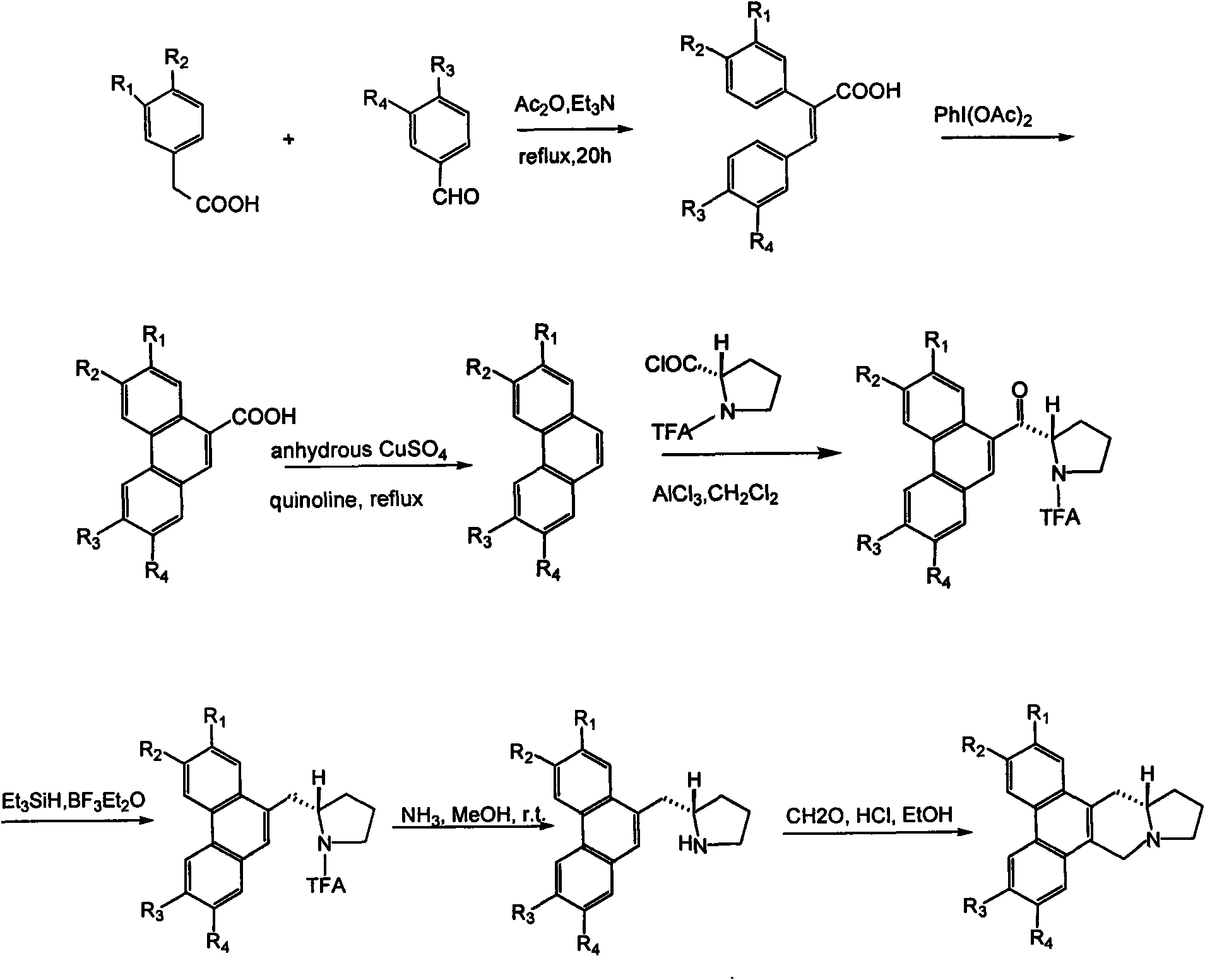
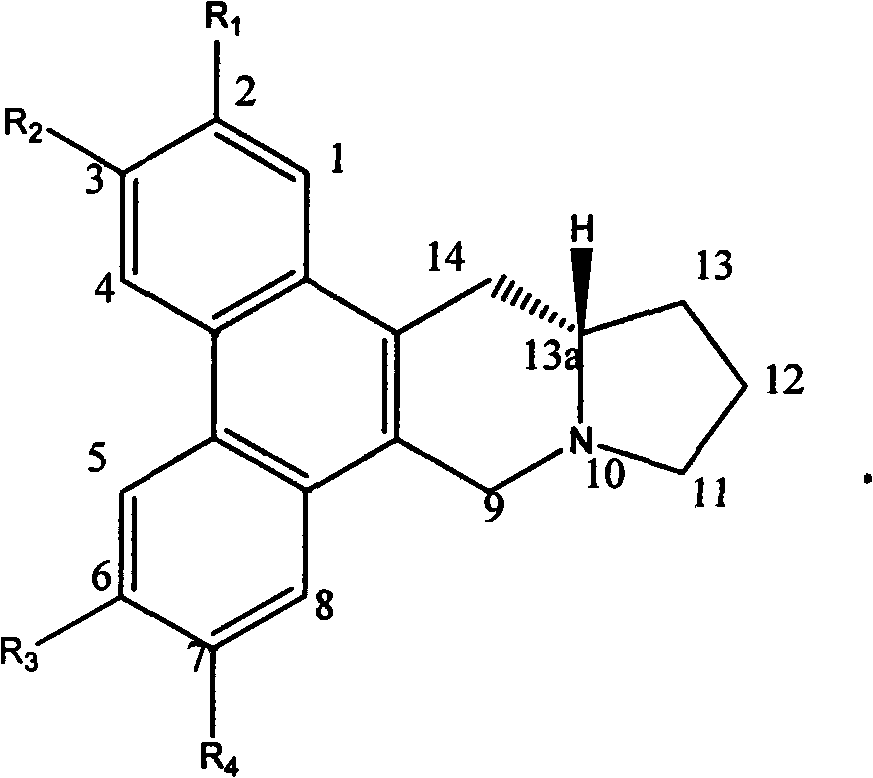
Tylophora ovata base analogs with anti-tumor activity and preparation method and use thereof
A technology of anti-tumor activity and seraphine, which is applied in the direction of anti-tumor drugs, organic active ingredients, medical preparations containing active ingredients, etc., can solve the problems of low alkaloid content, few plant resources, poor stability, etc., and achieve Simple synthetic route, significant inhibitory effect, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Inhibition of TDH on the proliferation of human cervical cancer cell line Hela
[0025] Get the human cervical cancer cell line Hela in the logarithmic growth phase, digest with trypsin, centrifuge at 1000r / min for 5min, count with trypan blue staining (percentage of living cells > 95%), and use 5×10 4 Cell density per ml was seeded in 96-well culture plate, 180 μl / well, cultivated for 24 hours, and added different concentrations of 2,3-dimethoxy-6,7-methylenedioxy-phenanthrene-[9,10- b]-indolizidine (TDH) (the drug is dissolved in DMSO, and the final concentration of DMSO in the system 50 The value is 4.05±0.22, which is only 50% of that of seraphine.
Embodiment 2
[0027] Inhibition of BMPI on the proliferation of human lung adenocarcinoma cell line A549
[0028] Get the human lung adenocarcinoma cell line A549 in the logarithmic growth phase, digest with trypsin, centrifuge at 1000r / min for 5min, count with trypan blue staining (percentage of viable cells > 95%), and measure 5×10 4 Cell density per ml was inoculated in 96-well culture plate, 180 μl / well, cultivated for 24 hours, and added different concentrations of 2,3,6,7-dimethylenedioxy-phenanthrene-[9,10-b]-indole Dorisidine (BMPI) (the drug is dissolved in DMSO, and the final concentration of DMSO in the system <0.1%, this concentration has no effect on cell growth), so that the final mass concentration is 0.25, 0.5, 1.0, 2.5, 5.0 μg / ml. There are 5 replicate wells in each group. At the same time, a solvent control group, namely a negative control group (containing DMSO required to dissolve the drug at the maximum concentration), and a zeroing group (containing an equal volume o...
Embodiment 3
[0030] Effects of BMPI on A549 Cell Division and Colony Formation Ability
[0031] Take A549 cells in the logarithmic growth phase, digest with 0.5% trypsin and blow into single cells, suspend the cells in RPMI-1640 culture medium containing 10% fetal bovine serum, count, and dilute the cell concentration with culture medium to 1 ×10 3 cells / ml, seeded in a six-well plate, 1×10 per well 3 cells, the total volume in the well is 2ml, shake well and place in CO 2In the incubator, incubate at 37°C for 24 hours, remove the culture medium, add BMPI (dissolved in DMSO, the final concentration of DMSO is less than 0.1%), the final concentration is 0.25, 0.5, 1.0, 2.5, 5.0 μg / ml. At the same time, a negative control group was set up. Shake well and incubate in a carbon dioxide incubator for 24 hours, remove the liquid medicine, replace with fresh culture medium, and continue to cultivate for 10 days. Discard the culture medium, wash twice with phosphate buffer (0.01mol / L, pH 7.4), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com