Method for synthesizing trinitrophloroglucinol
A technology of trinitrophloroglucinol and phloroglucinol is applied in the synthesis field of inactive energetic material precursor trinitrophloroglucinol), and can solve the problem of corrosion equipment, complicated reaction operation, large mixed acid, etc. problems, to achieve the effect of short reaction time, easy availability of raw materials and easy handling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
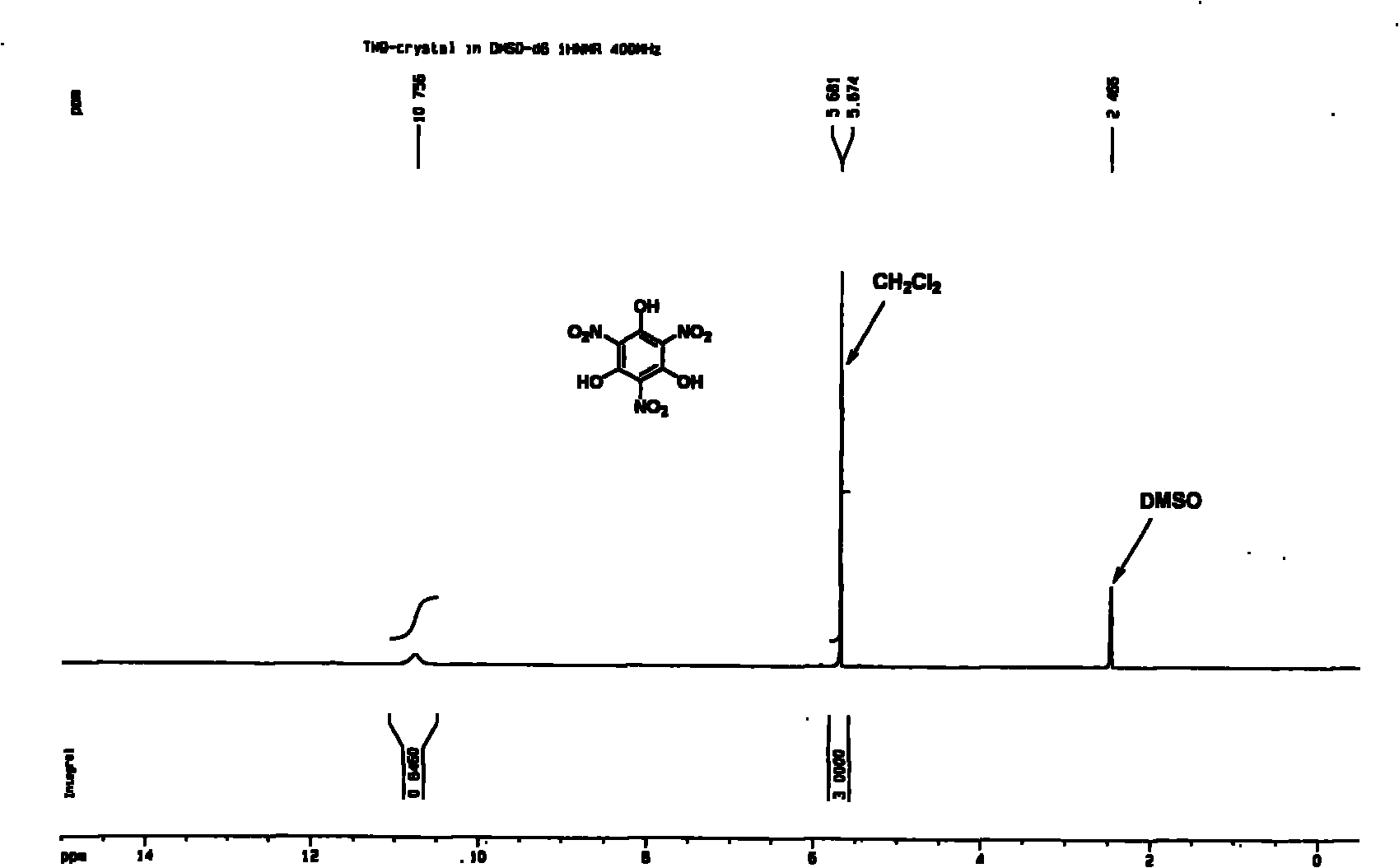
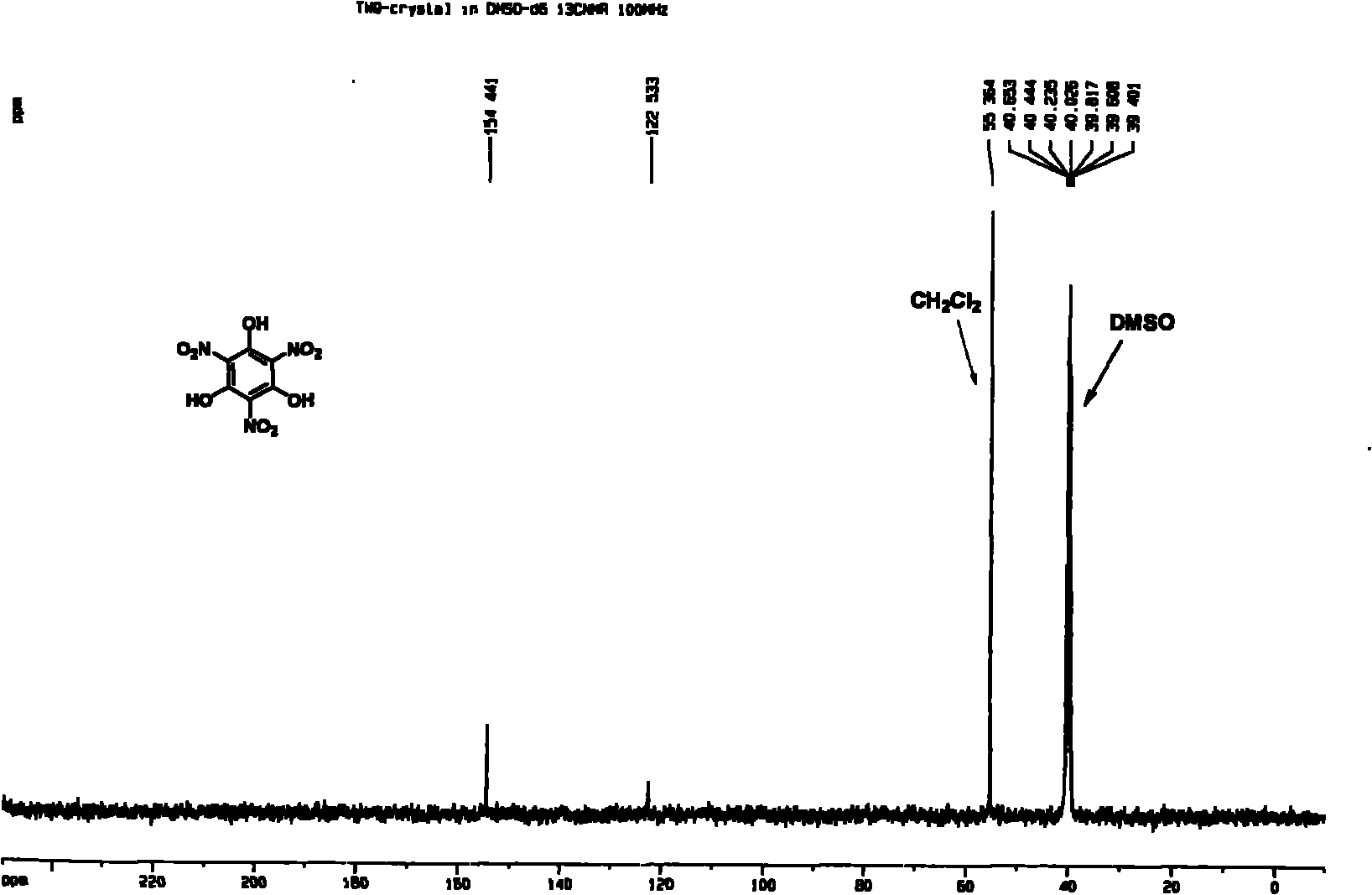
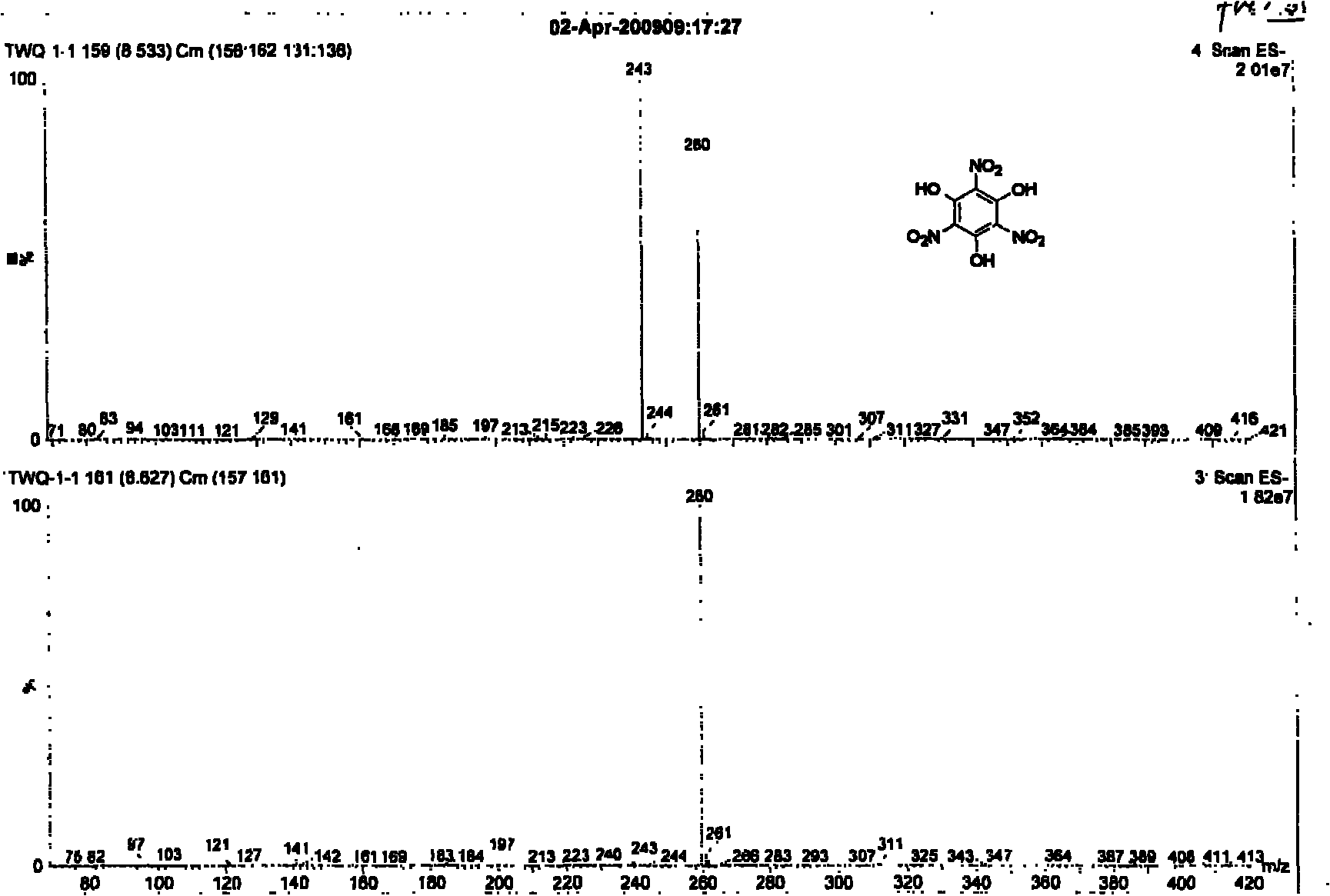
[0030] Weigh phloroglucinol (0.63g, 0.50mmol) and ZrO (NO 3 ) 2 2H 2 O (4.80g, 1.50mmol) in 50mL round bottom reaction flask, add 1.0g [Hmim] [HSO 4 ] ionic liquid, stirred evenly at room temperature, reacted in an oil bath at 50°C, cooled in an ice bath after initiation, added water to dilute after reacting at room temperature for 30 minutes, extracted with ethyl acetate (3 × 20mL) and concentrated under reduced pressure, TLC analysis (acetic acid Ethyl ester: acetone = 2: 1, R f =0.5), separated by flash column chromatography (ethyl acetate: acetone = 2: 1), the product 1,3,5-trihydroxy-2,4,6-trinitrobenzene (TNPG) was obtained as light yellow crystals. The NMR spectrum of the product is as figure 1 and figure 2 As shown, the mass spectrum is as image 3 shown.
Embodiment 2
[0032] Weigh phloroglucinol (0.63g, 0.50mmol) and Bi(NO 3 ) 3 ·5H 2 O (9.70g, 2.00mmol) in 50mL round bottom reaction flask, add 2.0g [Heim] [HSO 4 ] ionic liquid, after stirring evenly at normal temperature, trigger the reaction at 60°C in an oil bath, move to normal temperature for 30 minutes after triggering, add water to dilute, extract with ethyl acetate (3 × 20mL) and concentrate under reduced pressure, TLC analysis (ethyl acetate Ester: acetone = 2:1, R f =0.5), separated by flash column chromatography (ethyl acetate: acetone = 2: 1), the product 1,3,5-trihydroxy-2,4,6-trinitrobenzene (TNPG) was obtained as light yellow crystals.
Embodiment 3
[0034] Weigh phloroglucinol (0.63g, 0.50mmol) and VO (NO 3 ) 2 (6.32g, 2.50mmol) in a 50mL round bottom reaction flask, add 3.0g [Hbim] [HSO 4 ] ionic liquid, stirred evenly at room temperature, reacted in an oil bath at 70°C, cooled in an ice bath after initiation, added water to dilute after reacting at room temperature for 1 hour, extracted with ethyl acetate (3 × 20mL) and concentrated under reduced pressure, TLC analysis (acetic acid Ethyl ester: acetone = 2: 1, R f =0.5), separated by flash column chromatography (ethyl acetate: acetone = 2: 1), the product 1,3,5-trihydroxy-2,4,6-trinitrobenzene (TNPG) was obtained as light yellow crystals.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com