New method for preparing 1,2-pentadiol
A technology of pentanediol and pentanol, which is applied in the fields of hydrolysis preparation and organic chemistry, can solve the problems of heavy environmental pollution, difficult use, complex process, etc., and achieve the effect of reducing raw material cost, simple process, and wide source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
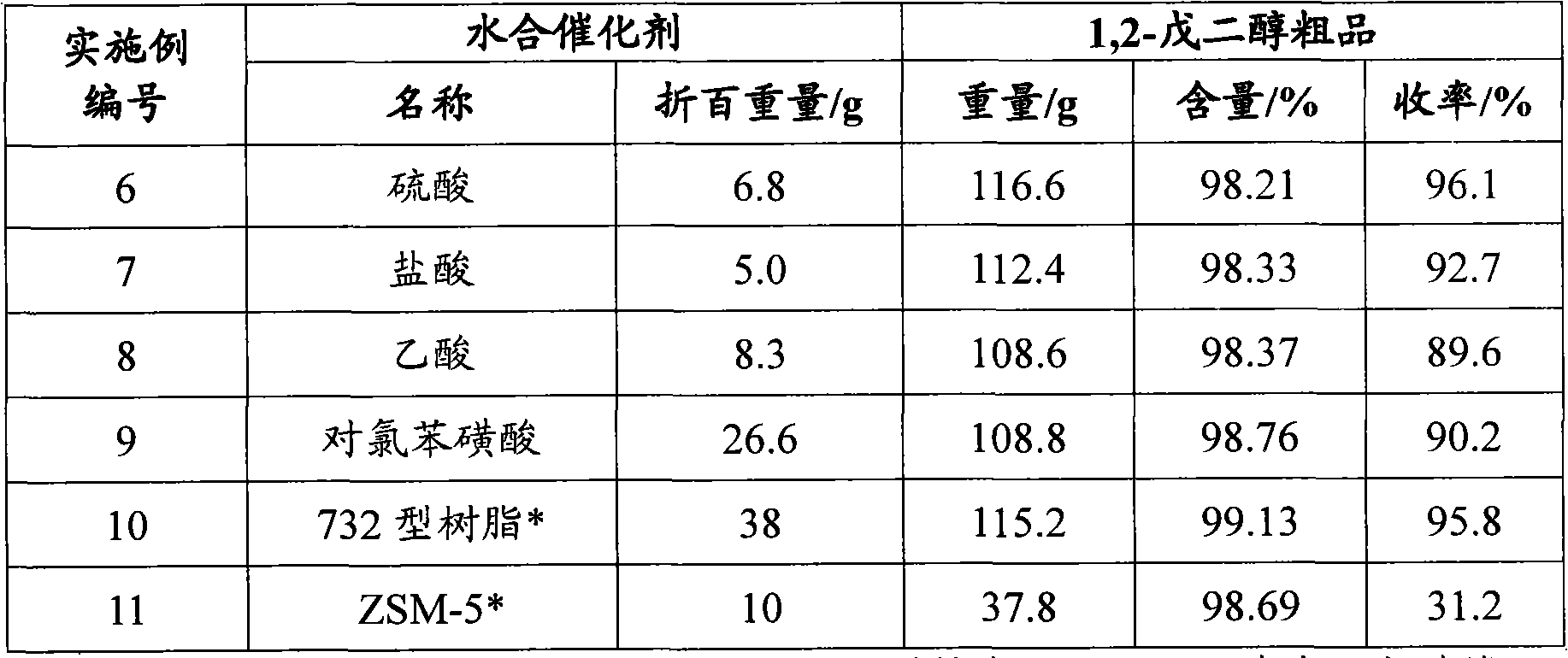
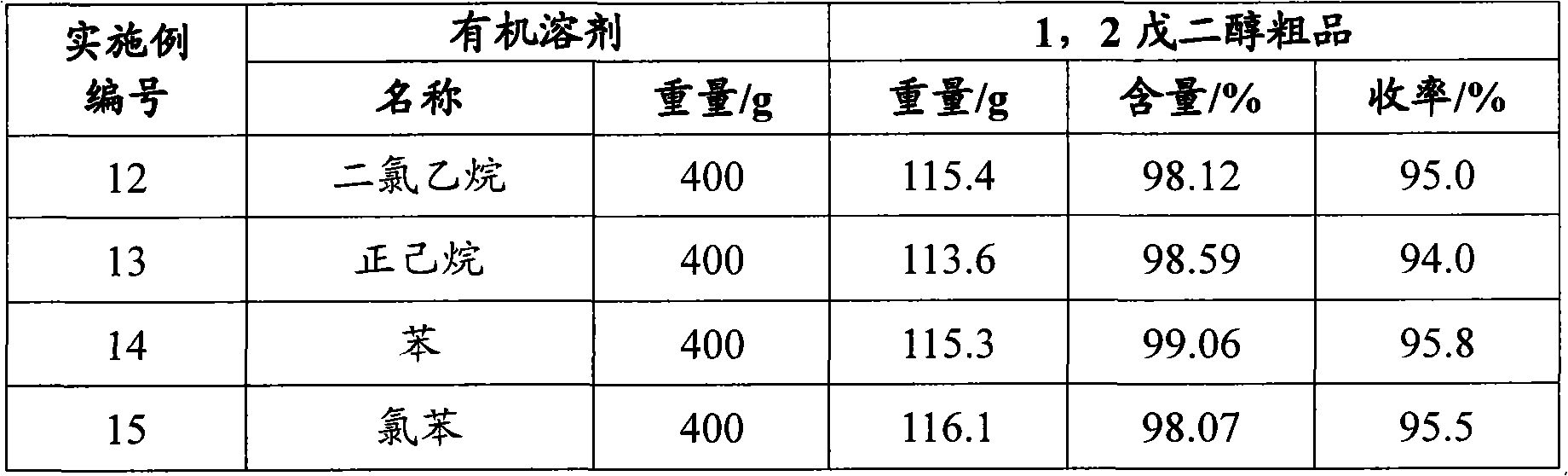
Embodiment 1
[0028] Embodiment 1 intermediate 1, the preparation of 2-epoxypentane
[0029] Add 555.7 g of chloropentanol (wherein the content of 2-chloro-1-pentanol is 99.0%, and 1-chloro-2-pentanol is 0.2%) in a four-necked glass flask equipped with a stirrer, a condenser, and a thermometer 10% sodium hydroxide aqueous solution 2250g, start stirring, heat up to 70°C, heat preservation reaction for 60min; drop to room temperature, neutralize the pH to 7 with hydrochloric acid, let stand, and separate layers to obtain 382.7g of the upper yellow oily substance which is 1, 2-Epoxypentane, its purity is 98.6%, and the yield of p-chloropentanol is 97.5%.
Embodiment 2
[0030] Embodiment 2 intermediate 1, the preparation of 2-epoxypentane
[0031] Add 562.5 g of chloropentanol (wherein the content of 2-chloro-1-pentanol is 68.6%, 1-chloro-2-pentanol 29.4%) in the four-necked glass flask equipped with stirrer, condenser and thermometer , 2250g of 10% aqueous sodium hydroxide solution, started stirring, raised the temperature to 70°C, and kept the temperature for 60 minutes; lowered to room temperature, neutralized the pH to 7 with hydrochloric acid, allowed to stand, and separated into layers to obtain 383.4g of the upper yellow oily substance which was 1, 2-Epoxypentane, its purity is 96.8%, the yield of p-chloropentanol is 95.9%.
Embodiment 3
[0032] Embodiment 3 intermediate 1, the preparation of 2-epoxypentane
[0033] Add 554.0 g of chloropentanol (wherein the content of 2-chloro-1-pentanol is 0.3%, 1-chloro-2-pentanol 99.2%) in the four-necked glass flask equipped with stirrer, condenser and thermometer 10% sodium hydroxide aqueous solution 2250g, start stirring, heat up to 70°C, keep warm for 60min; cool down to room temperature, neutralize the pH to 7 with hydrochloric acid, let stand, separate layers, and obtain 385.0g of the upper yellow oily substance which is 1, 2-Epoxypentane, its purity is 97.2%, the yield of p-chloropentyl alcohol is 96.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com