Precipitation and crystallization drug sustained and controlled release microspheres and preparation method thereof
A technology for drugs and microspheres, which is applied in the preparation field of precipitation crystallization drug sustained and controlled release microspheres and precipitation crystallization drug sustained and controlled release microspheres, can solve the problem of irregular shape, negative impact on production time and cost, and drug release. speed difference and other problems, to achieve the effect of improving compressibility, improving the fluidity of dry powder and shortening time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
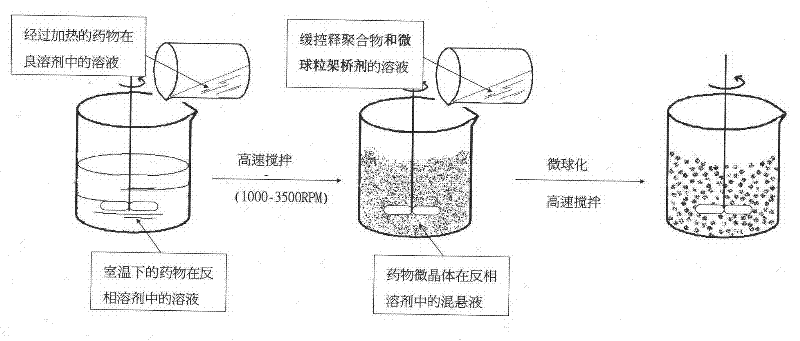
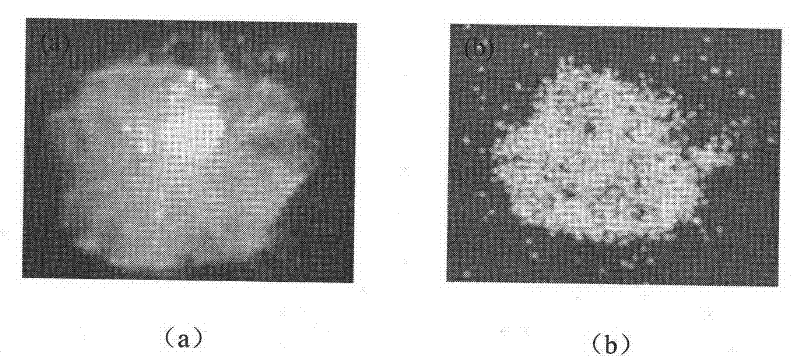
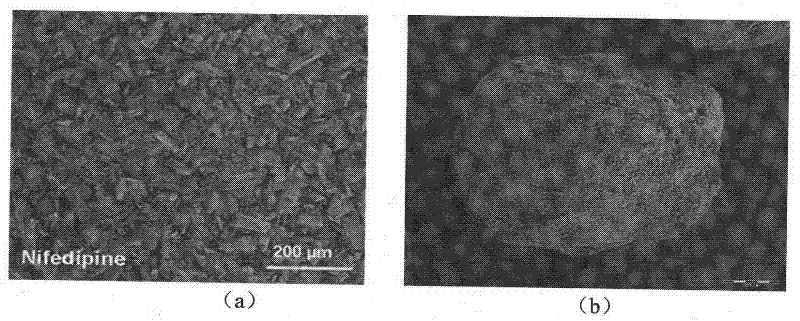
[0038] A 1% (W / V) solution of nifedipine in acetone was prepared by heating to 55°C. Then heating and fully dissolving the acetone solution of clarified nifedipine under high-speed stirring (1000RPM) and at room temperature is added to purified water (the volume ratio of the acetone solution of nifedipine to purified water is 1: 500), and in Wait for 0.5 hour under high-speed stirring, until the microcrystals of nifedipine are completely precipitated, and the good solvent acetone of nifedipine basically volatilizes. Afterwards, the solution of the pre-prepared microsphere bridging agent (in this embodiment, eucalyptol) and the drug sustained and controlled release polymer (in this embodiment, acrylic resin RL) in chloroform (in this solution, slow control The concentration of release polymer is 10%, the concentration of microspheroid bridging agent is 15%) slowly under high-speed stirring (1000RPM) and join in the microcrystal suspension that nifedipine microcrystal forms in w...
Embodiment 2
[0047] A solution of 20% (W / V) nifedipine in acetone was prepared by heating to 55°C. Then heating and fully dissolving the acetone solution of clarified nifedipine under high-speed stirring (4000RPM) and at room temperature is added to purified water (the volume ratio of the acetone solution of nifedipine to purified water is 1: 5), and in Wait for 2 hours under high-speed stirring until the microcrystals of nifedipine are completely precipitated, and the good solvent acetone of nifedipine basically volatilizes. Afterwards, the solution of microsphere bridging agent prepared in advance (using peppermint oil in this embodiment) and drug sustained and controlled release polymer (acrylic resin RS in this embodiment) in chloroform (sustained and controlled release in this solution) The concentration of the polymer is 1%, the concentration of the microspheroid bridging agent is 10%) slowly under high-speed stirring (4000RPM) into the microcrystal suspension formed by the nifedipin...
Embodiment 3
[0049] A 10% (W / V) solution of nifedipine in acetone was prepared by heating to 55°C. Then heating and fully dissolving the acetone solution of clear nifedipine under high-speed stirring (2000RPM) and at room temperature is added to purified water (the volume ratio of the acetone solution of nifedipine to purified water is 1: 1000), and in Wait for 1 hour under high-speed stirring until the microcrystals of nifedipine are completely precipitated, and the good solvent acetone of nifedipine basically volatilizes. Afterwards, the solution of the pre-prepared microsphere bridging agent (using olive oil in this embodiment) and the drug sustained and controlled release polymer (using polylactic acid-lactide in this embodiment) in chloroform (in this solution The concentration of slow and controlled release polymer is 40%, and the concentration of microspheroid bridging agent is 35%) slowly under high-speed stirring (2000RPM) joins in the microcrystal suspension that nifedipine micro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com