Long-acting ceftiofur hydrochloride injection and preparation method thereof
A technology of ceftiofur hydrochloride and injection, which is applied in the direction of medical preparations containing active ingredients, liquid delivery, pharmaceutical formulas, etc., can solve the problems of inconvenience in veterinary treatment and incompatibility in treatment of animals, and achieve simple preparation methods, Good stability, less irritation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
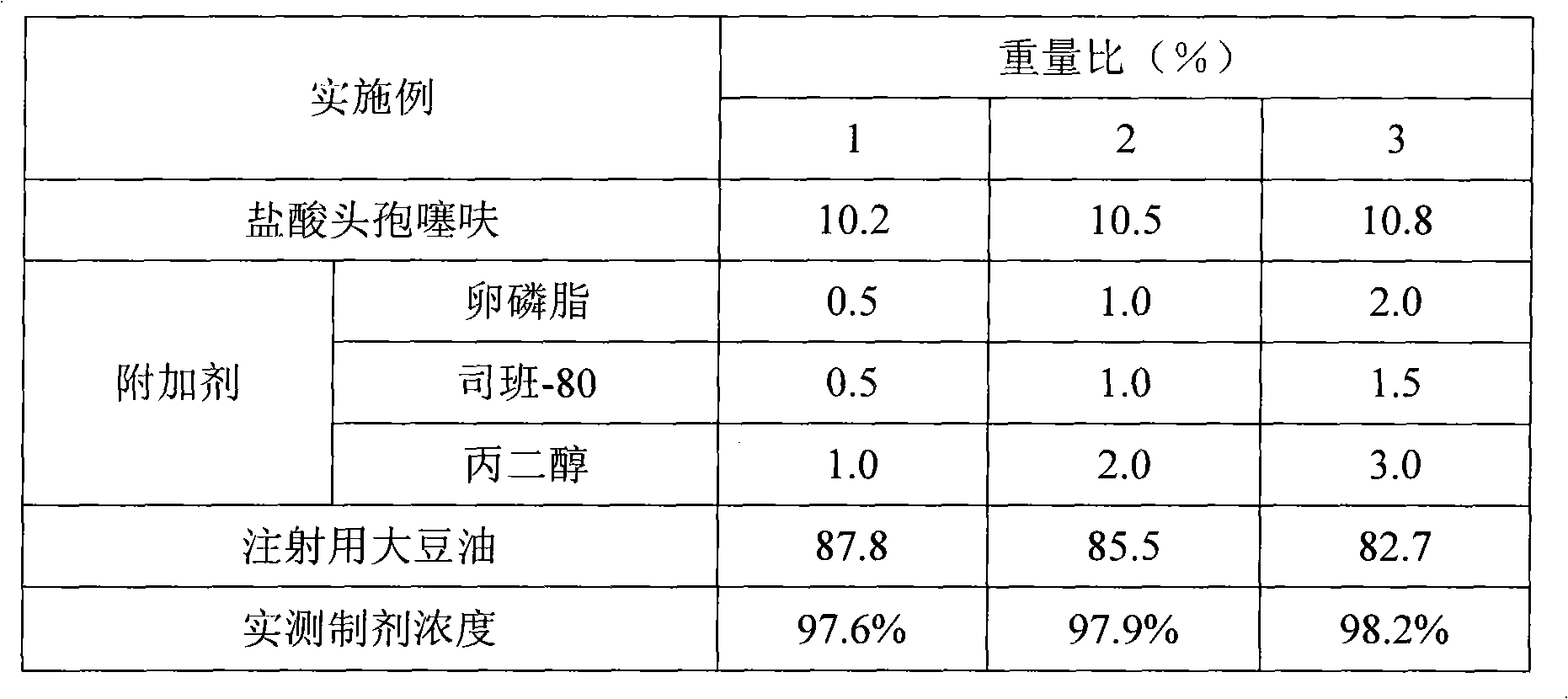
[0026] Example 1-3 , Preparation of long-acting ceftiofur hydrochloride injection
[0027] Prepare long-acting ceftiofur hydrochloride injection according to the formula shown in Table 1, and the specific operations are as follows:
[0028] Heat soybean oil for injection to 90°C in a sterilized container, then add additives Span-80, propylene glycol, and lecithin, stir continuously to mix and dissolve, continue heating to 150°C, and sterilize for 1 hour. After cooling, add ceftiofur hydrochloride, continue to stir for 20 minutes, supplement soybean oil for injection to 100% (1000ml), use a GZJ type high-shear homogenizer to stir and mix evenly, and import the suspension into a 10,000-class clean room for filling. Pack in 10ml / 50ml molded glass bottles, cover with butyl rubber stopper and aluminum cap, crimp the cap, and pack after light inspection.
[0029] During the preparation process, the purpose of using GZJ type high shear homogenizer for homogenization operation is to...
Embodiment 4
[0032] Example 4 ,clinical experiments
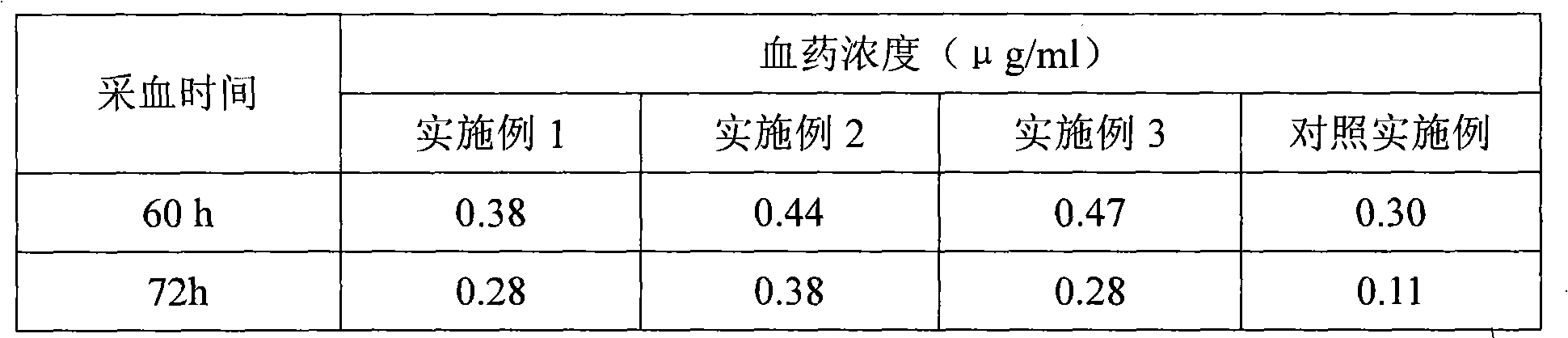
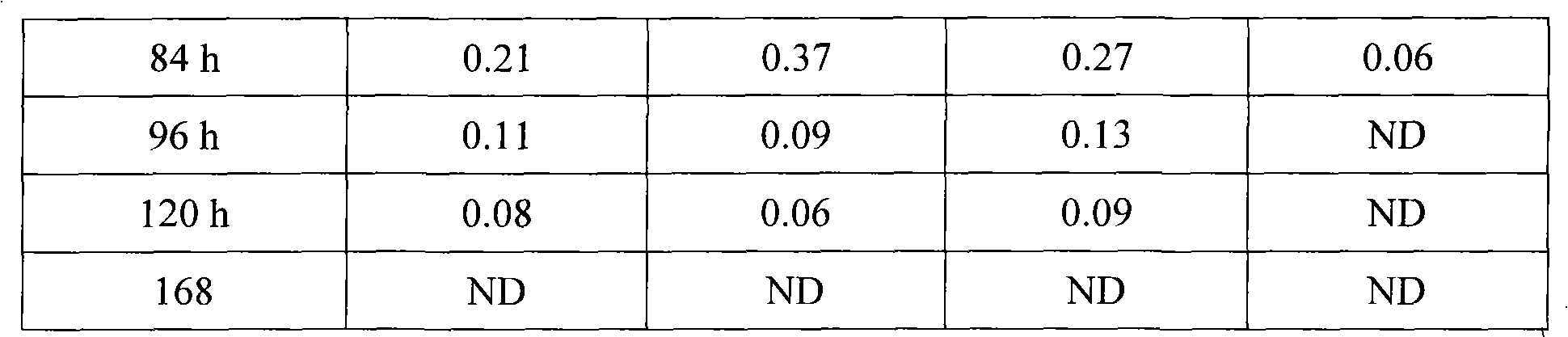
[0033] Get 12 pigs (body weight 30~40 kilograms, male and female half and half), divide into three groups at random, inject the long-acting ceftiofur hydrochloride injection prepared in embodiment 1~3 by intramuscular injection of 5 mg per kilogram of body weight, and inject cephalosporin hydrochloride Thiofur (Shandong Lukang Pharmaceutical Co., Ltd., batch number 071103) was used as a control example, and the intravenous injection of ceftiofur hydrochloride was used as a bioavailability control, and then blood was collected from the axillary vein to detect the blood drug concentration, and the following tests were performed.
[0034] 4.1. Detection of drug concentration in plasma
[0035] After the pigs were injected with the drug, blood was collected from the axillary vein, and the concentration of ceftiofur in the plasma was detected at various time points by ion exchange-high performance liquid chromatography. The results are sho...
Embodiment 5
[0048] Example 5 , Product stability experiment
[0049] The long-acting ceftiofur hydrochloride injection described in Examples 1 to 3 was stored at an ambient temperature of 30°C. After 6 months of observation, it was found that the product remained a milky yellow oil suspension. After 10 days of strong light irradiation, The product stays the same color.
[0050] According to the relevant regulations in the appendix of "The Veterinary Pharmacopoeia of the People's Republic of China" 2005 edition, the main drug content, sedimentation volume ratio, and accelerated test of the long-acting ceftiofur hydrochloride injection described in Examples 1 to 3 are detected, specifically See Table 4.
[0051] Table 4
[0052]
[0053] As can be seen from Table 4, the content of the main drug, sedimentation volume ratio, release rate, accelerated test and other aspects of the long-acting ceftiofur hydrochloride injection of the present invention all meet the standards of the Chines...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com