Mycoplasma clearing reagent and application thereof
A technology of mycoplasma and reagent, applied in the field of mycoplasma removal reagent, can solve the problems of different, easy repeated infection of mycoplasma and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Configuration of mycoplasma removal reagent
[0050] Prepare 250ul reagent according to the following formula (each component is calculated by weight percentage):
[0051] Formulation 1: Gemifloxacin 1.4%
[0052] Minocycline 0.4%
[0053] Azithromycin 0.2%
[0054] Buffer 98%
[0055] Buffer formula:
[0056] NaOH 0.4%
[0057] Nacl 0.9%
[0058] Glucose 5%
[0059] H 2 O 93.7%
[0060] Formulation 2: Moxifloxacin 1.3%
[0061] Doxycycline 0.5%
[0062] Clarithromycin 0.1%
[0063] Buffer 98.1%
[0064] Buffer formula:
[0065] NaOH 0.2%
[0066] Nacl 1.0%
[0067] Glucose 8%
[0068] H 2 O 90.8%
[0069] Formula 3: Clinfloxacin 1.5%
[0070] Tigecycline 0.3%
[0071] Roxithromycin 0.3%
[0072] Buffer 97.9%
[0073] Buffer formula:
[0074] NaOH 0.6%
[0075] Nacl 0.9%
[0076] Glucose 5%
[0077] H 2 O 93.5%
[0078] Configuration method
[0079] 1. Buffer configuration
[0080] 1) Ultra-pure water 103.4kpa steam pressure temperature reaches 121.3℃, maintain 15-20 minutes moist heat steriliza...
Embodiment 2
[0086] Example 2 Test of mycoplasma removal effect of mycoplasma removal reagent
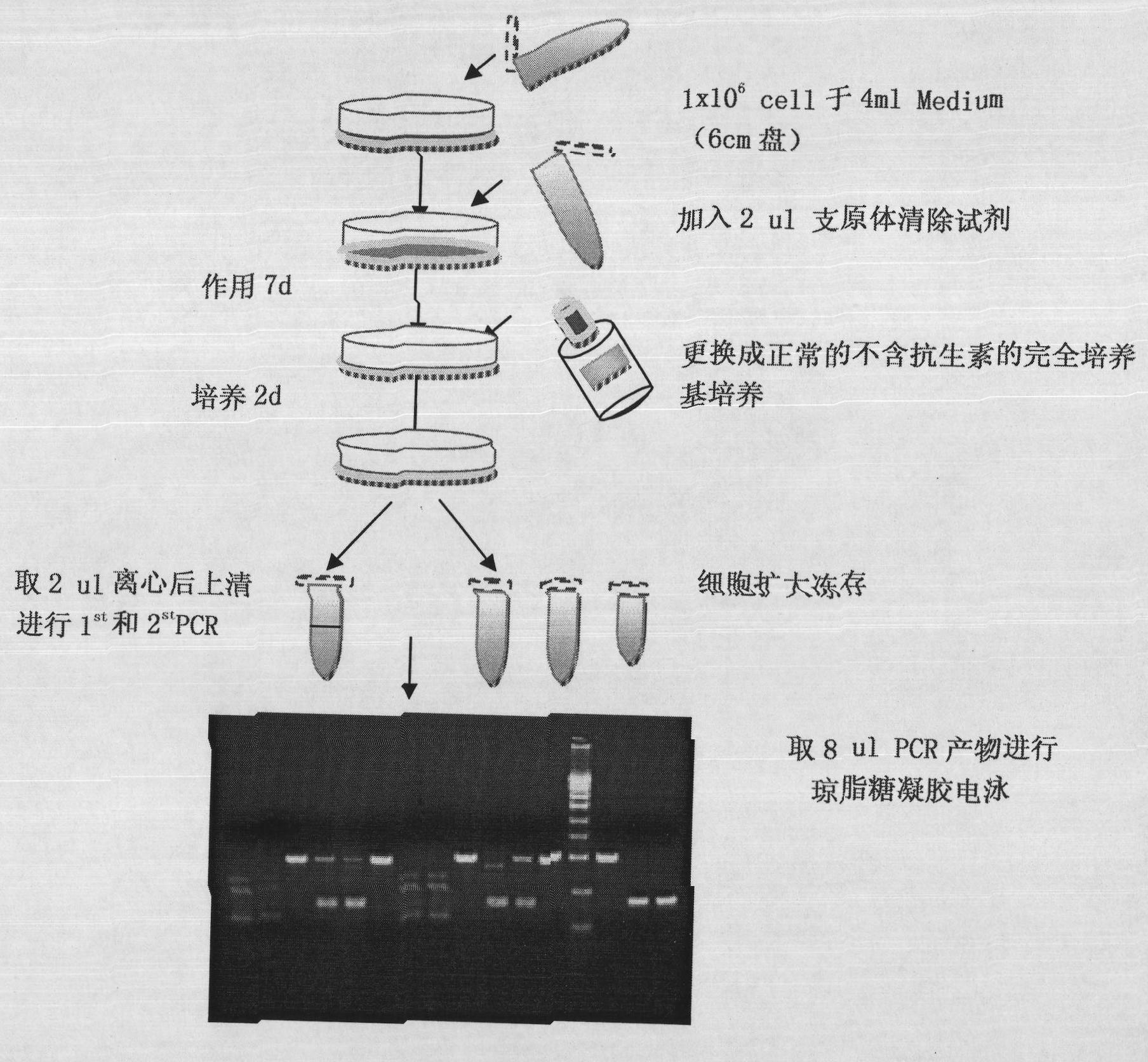
[0087] The mycoplasma scavenging reagent prepared by formula 1 in Example 1 was used as follows figure 1 Said includes the following steps:
[0088] 1. Resuscitate the cells, plant the plate after adjustment, and plant 1x10 in a 6cm plate 6 Cells
[0089] 2. After the cells adhere to the wall the next day, dilute the mycoplasma removal reagent into a medium containing 5% FBS without dual antibody at a ratio of 2000x, and use this medium for culture during subsequent passages;
[0090] 3. After acting for 7 days, pay attention to observe and record the cell status during this process, and use the medium containing mycoplasma scavenging reagent to maintain when changing the medium or passage;
[0091] After 4.7 days, change to normal complete medium without antibiotics and culture for 2 days, and increase the volume of each plate to 4-5ml;
[0092] After 5.2 days, aspirate 500ul of culture supernatant, and p...
Embodiment 3
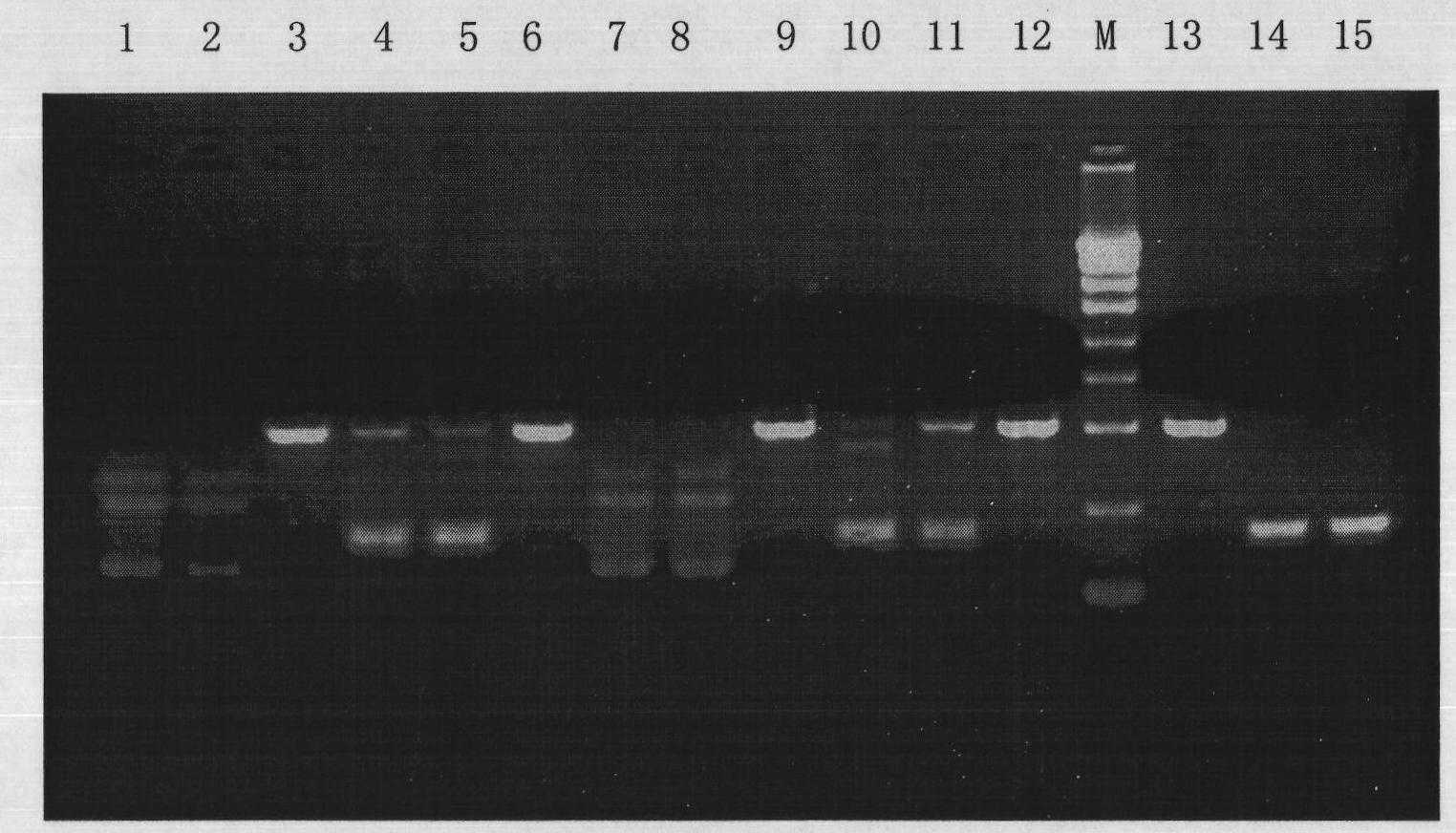
[0096] Example 3 Mycoplasma scavenging effect test of reagent
[0097] experiment equipment:
[0098] Ultrasound stage, water bath, centrifuge, negative 80 degree refrigerator, PCR machine, electrophoresis tank, gel imager, electrophoresis,
[0099] Sterile PCR tube Sterile 1.5ml EP tube PCR tube rack Sterile pipette tip Cotton ball
[0100] Pipette (1ml 200ul 10ul 2ul)
[0101] Experimental reagents:
[0102] Sterile ultrapure water Sample to be tested 75% sterile alcohol Mycoplasma spray (prepared in Example 1)
[0103] Germ-free test medium
[0104] Nested PCR Mycoplasma Rapid Detection Kit (Shanghai Jisheng Pharmaceutical Company)
[0105] 2% agarose gel EB electrophoresis buffer Mark (DL250+) 6x Loading Buffer
[0106] Experimental steps:
[0107] Refer to the method of Example 2 to use a mycoplasma scavenging reagent.
[0108] Use the following method to use the nested PCR Mycoplasma rapid detection kit:
[0109] 1. Collect the samples to be tested and store in the refrigerator at minus 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com