Method for preparing methyl phenyl hydrogen-containing silicone oil by rare earth super acid catalysis
A technology of methylphenyl cyclosiloxane and methylphenyl, which is applied in the field of rare earth superacid catalyzed preparation of methylphenyl hydrogen-containing silicone oil, can solve problems such as pollution and complicated preparation process, and achieve simple process and labor-intensive Low, easy-to-operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
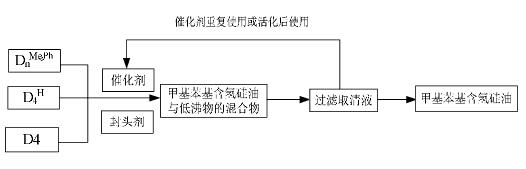
Image
Examples
Embodiment 1
[0027] in N 2 Add methylphenylcyclosiloxane monomer D to a 100mL dry three-neck flask under protection n Me,Ph (Mixtures of n=3, 4, 5 or 6 prepared by myself in any proportion) (34.50g, 0.2537mol of methylphenylsiloxane chain), D4 (27.10g, dimethylsiloxane chain section 0.3662mol) and D 4 H (0.40g, 0.0067mol of methyl hydrogen siloxane chain unit), remove the water in polysiloxane monomer at 35℃~45℃ / -0.096MPa, and then add end-capping agent 1,1,3, 3-Tetramethyl-1,3-dihydrodisiloxane (0.67g, 0.005mol), rare earth solid superacid TiO 2 / SO 4 2- / Nd 3+ (3.1g, 5% of the total monomer mass), at N 2 Under protection, polymerize at 100° C. for 5 h, and recover the catalyst by suction filtration. Reduce the pressure to -0.096MPa, gradually raise the temperature to 205°C to remove low-molecular compounds, stop heating when there is no fraction within 5 minutes, and cool to room temperature to obtain the product, whose refractive index is n D 25 =1.4950, dynamic viscosity 300...
Embodiment 2
[0029] in N 2 Add methylphenylcyclosiloxane monomer D to a 100mL dry three-neck flask under protection n Me,Ph (Mixtures of n=3, 4, 5 or 6 prepared by myself in any proportion) (40.55g, 0.2982mol of methylphenylsiloxane chain), D4 (21.50g, dimethylsiloxane chain section 0.2905mol) and D 4 H (0.4g, 0.0067mol of methyl hydrogen siloxane chain unit), remove the water in polysiloxane monomer at 35℃~45℃ / -0.096MPa, and then add end-capping agent 1,1,3, 3-Tetramethyl-1,3-dihydrodisiloxane (0.67g, 0.005mol), rare earth solid superacid TiO 2 / SO 4 2- / Nd 3+ (3.1g, 5% of the total monomer mass), at N 2 Under protection, polymerize at 100° C. for 5 hours, and recover the rare earth solid superacid catalyst by suction filtration. Reduce the pressure to -0.096MPa, gradually raise the temperature to 205°C to remove low-molecular compounds, stop heating when there is no fraction within 5 minutes, and cool to room temperature to obtain the product, whose refractive index is n D 25 ...
Embodiment 3
[0031] in N 2 Add methylphenylcyclosiloxane monomer D to a 100mL dry three-neck flask under protection n Me,Ph (Mixtures of n=3, 4, 5 or 6 prepared by myself in any proportion) (46.35g, 0.3408mol of methylphenylsiloxane chain), D4 (15.65g, dimethylsiloxane chain section 0.2115mol) and D 4 H (0.4g, 0.0067mol), remove the water in polysiloxane monomer at 35℃~45℃ / -0.096MPa, then add end-capping agent 1,1,3,3-tetramethyl-1 ,3-Dihydrodisiloxane (0.67g, 0.005mol), rare earth solid superacid TiO 2 / SO 4 2- / Nd 3+ (3.1g, 5% of the total monomer mass), at N 2 Under protection, polymerize at 100° C. for 5 hours, and recover the rare earth solid superacid catalyst by suction filtration. Reduce the pressure to -0.096MPa, gradually raise the temperature to 205°C to remove low-molecular compounds, stop heating when there is no fraction within 5 minutes, and cool to room temperature to obtain the product, whose refractive index is n D 25 =1.5120, dynamic viscosity 400mPa??S , thr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com