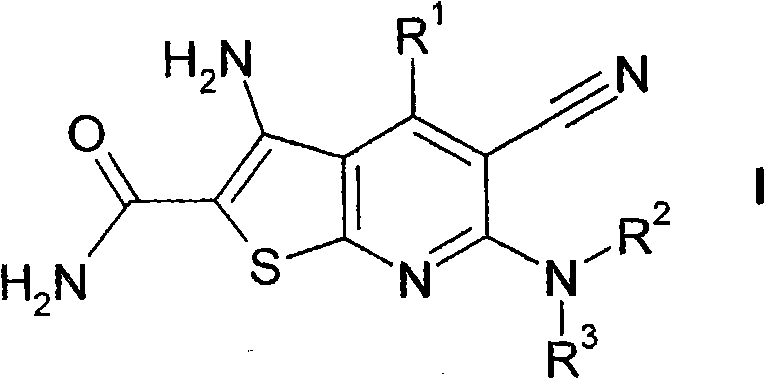
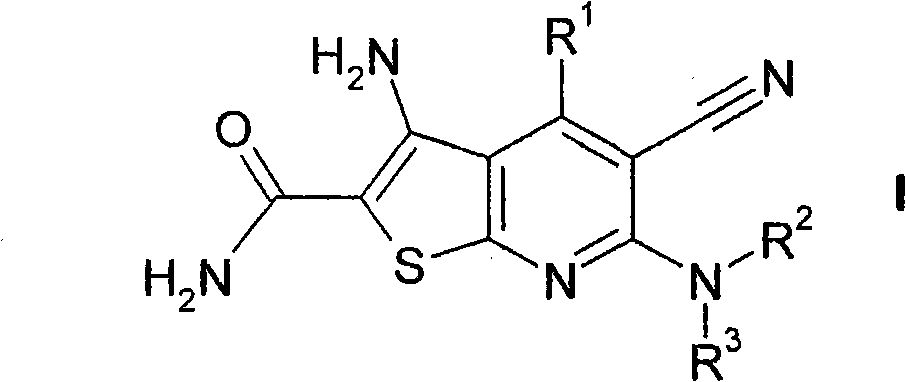
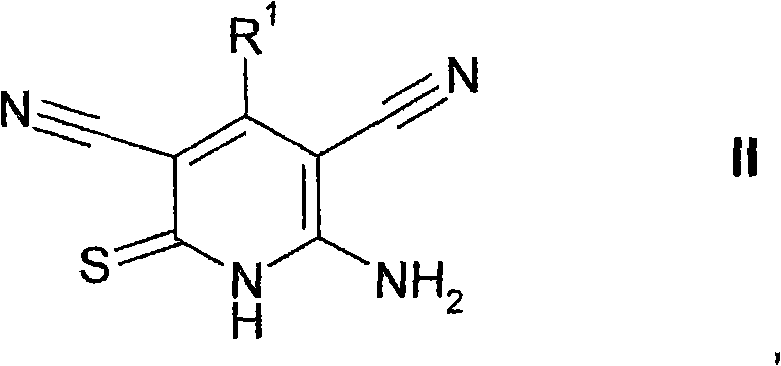
5-cyano-thienopyridines for the treatment of tumors
A technology of pyridine and cyano, which is applied in the field of new compounds for the treatment of kinase-induced diseases and the preparation of drugs, which can solve the problems of increasing tumor risk and accelerating tumor development, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0266] for the preparation of which R 2 and R 3 A general reaction scheme for compounds of formula I each representing H:
[0267]
[0268] Preparation of 3,6-diamino-4-benzofuran-2-yl-5-cyanothieno[2,3-b]pyridine-2-carboxamide ("A1"):
[0269] 1.1 First, 2.685 g of malononitrile and 4.196 g of cyanothioacetamide were added to a solution of 6.0 g of benzofuran-2-carbaldehyde ("E1") in 200 mL of ethanol. Then 203.2 μL of piperidine was added and the resulting red-brown suspension was boiled at 90° C. for 3 hours and subsequently stirred at room temperature for 16 hours. The precipitated material (yellow and orange crystals) was separated, washed with ethanol and dichloromethane and dried to give 8.1530 g of 6-amino-4-benzofuran-2-yl-2-thio-1,2-dihydro A mixture of pyridine-3,5-dicarbonitrile and 2,6-diamino-4-benzofuran-2-yl-2H-thiopyran-3,5-dicarbonitrile (“1”), which may not be purified And react further.
[0270] 1.2 To a solution of 1.0 g of the mixture ("1") prepar...
Embodiment 2
[0302]
[0303] 3-amino-4-benzofuran-2-yl-5-cyano-6-[3-(4-methyl-piperazin-1-yl)propylamino]thieno[2,3-b] Preparation of pyridine-2-carboxamide ("A30"):
[0304] 2.1 Under an inert atmosphere, 8.14 g ("2") was added to a solution of 3.97 g copper(II) chloride and 4.96 mL isoamyl nitrite in 470 mL dry acetonitrile. The resulting suspension was heated to 65°C and stirred for 4 hours. The resulting red-brown solution was cooled to room temperature, transferred to 400 mL hydrochloric acid (20% by weight), and extracted 3 times with 100 mL each of ethyl acetate. The combined organic phases were concentrated and transferred to ice water. The resulting precipitate was isolated, washed with acetonitrile and water and dried to give 3.1326 g of 2-(4-benzofuran-2-yl-6-chloro-3,5-dicyanopyridin-2-ylsulfanyl) Acetamide ("3") sandy powder.
[0305] HPLC content: 95.8%
[0306] HPLC-MS: [M+H]369
[0307] 2.2 At room temperature, 200 mg of 2-(4-benzofuran-2-yl-6-chloro-3,5-dicyanopyr...
Embodiment 3
[0347] If steps 1.1 and 1.2 of Example 1 are carried out analogously using furan-2-carbaldehyde as "E1", 2-(6-amino-4-furan-2-yl-3,5-dicyano-pyridine- 2-ylsulfanyl)acetamide ("2a"). "2a" is subsequently reacted analogously to steps 2.1 to 2.3 of Example 2 using 3-pyrrolidin-1-ylpropylamine as "E2" in step 2.2. 3-amino-5-cyano-4-furan-2-yl-6-(3-pyrrolidin-1-ylpropylamino)thieno[2,3-b]pyridine-2-carboxamide "A51 ".
[0348]
[0349] HPLC-MS: [M+H]411
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com