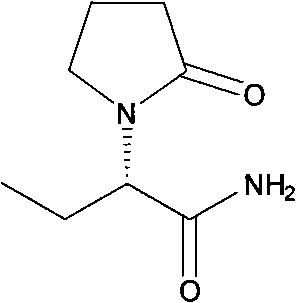
Method for preparing L-2-aminobutyric acid by enzyme method
A technology for aminobutyric acid and enzymatic preparation, applied in the direction of fermentation, etc., can solve the problems of low process yield, high cost, unsuitable process conditions, etc., and achieve the effects of low process cost, mild reaction conditions, and easy directional control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Add 20mg L-threonine, 32mg ammonium formate (about 2.5 times equivalent), 0.2mgNAD to the 5ml reaction bottle + and 2ml phosphate buffered saline (Na 2 HPO 4 / NaH 2 PO 4 , 0.1M, pH 7.5), adjust the pH value of the solution to 7.5 with concentrated ammonia water, then add 1mg of threonine deaminase crude enzyme, 2mg of leucine dehydrogenase crude enzyme and 2mg of formate dehydrogenase crude enzyme, in After 3 hours of magnetic stirring reaction at 25°C, samples were taken for HPLC analysis, and the conversion rate>95% (C18 column; UV 200nm detection; mobile phase: 20mM phosphate buffer (pH 3.0) / acetonitrile=95 / 5, v / v ), ee value>99% (CR (+) column; UV 200nm detection; mobile phase: perchloric acid aqueous solution (pH 1.5)).
Embodiment 2
[0016] Add 50mg L-threonine, 80mg ammonium formate (about 2.5 times the equivalent), and 2.5mg NAD to a 25ml Erlenmeyer flask + and 5ml phosphate buffered saline (Na 2 HPO 4 / NaH 2 PO 4 , 0.1M, pH 8.0), adjust the pH value of the solution to 8.0 with concentrated ammonia water, then add 5mg of threonine deaminase crude enzyme, 10mg of leucine dehydrogenase crude enzyme and 20mg of formate dehydrogenase crude enzyme, in After reacting on a rotary shaker at 160 rpm at 37° C. for 20 hours, samples were taken for HPLC analysis. The conversion rate was >99%, and the ee value was >99%.
Embodiment 3
[0018] Add 150mg L-threonine, 480mg ammonium formate (about 2.5 times the equivalent), 2.5mgNAD in a 25ml Erlenmeyer flask + and 5ml phosphate buffered saline (Na 2 HPO 4 / NaH 2 PO 4 , 0.1M, pH 7.0), adjust the pH value of the solution to 7.0 with concentrated ammonia water, then add 2.5mg of threonine deaminase crude enzyme, 1mg of leucine dehydrogenase crude enzyme and 5mg of formate dehydrogenase crude enzyme, After reacting on a rotary shaker at 160 rpm at 30° C. for 24 hours, samples were taken for HPLC analysis. The conversion rate was about 65%, and the ee value was >99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com