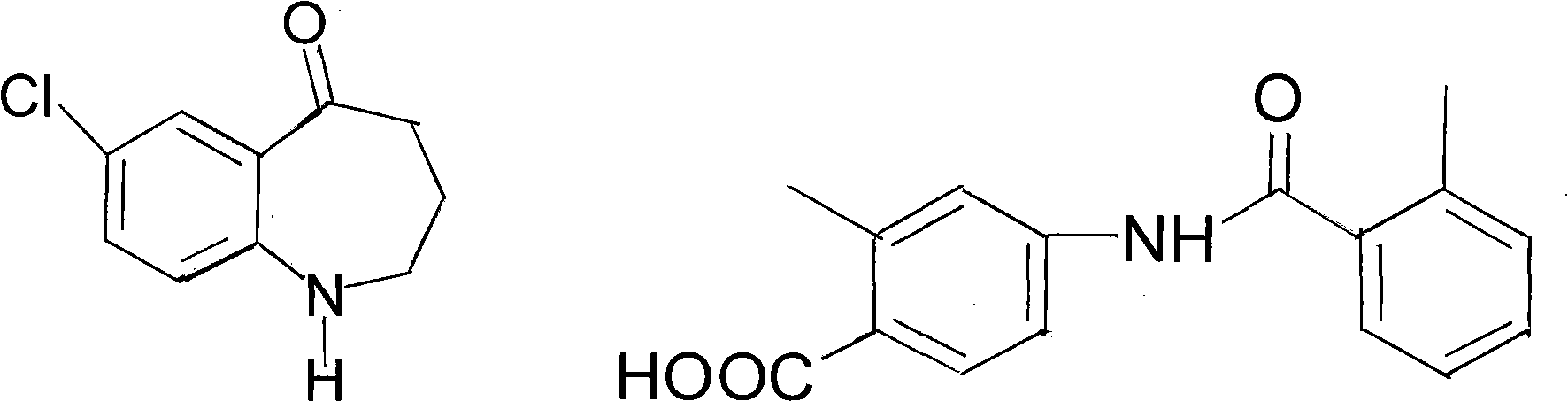
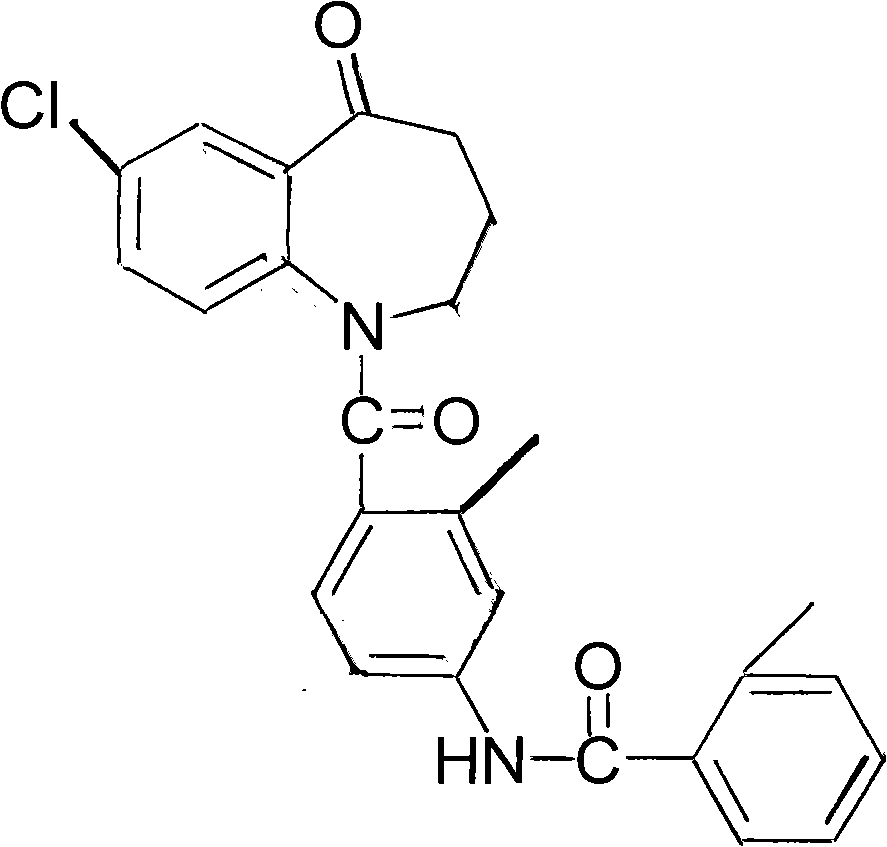
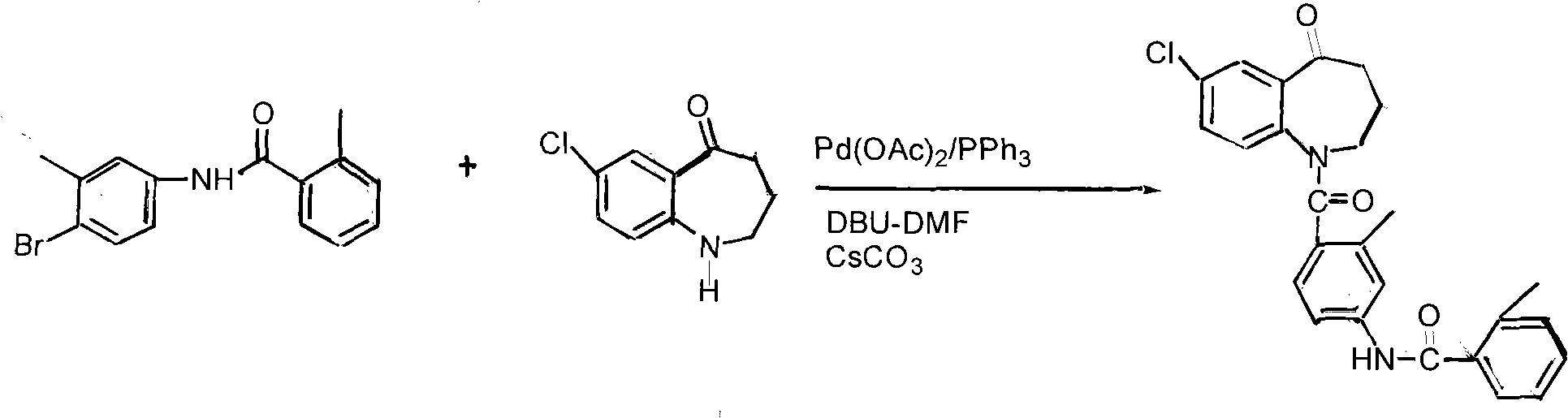
Method for preparing tolvaptan intermediate
A technology of tolvaptan and intermediates, which is applied in the field of drug synthesis and can solve problems such as difficult industrial production, difficulty in industrial production, and difficult amidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 6.4g (0.0238mol) of the general formula (3) into a 100ml four-necked bottle, add 60ml tetrahydrofuran, stir, and it becomes a cloudy liquid, add 1 drop of DMF, raise the temperature to 40°C and start adding 3.4g (0.0286mol) of thionyl chloride dropwise ), dropped in about 10 minutes, kept at 40°C, and reacted for 4 hours (TLC tracking, until the raw material point disappeared), evaporated the solvent under reduced pressure to obtain a brownish yellow oil; dissolved in 40ml of dichloromethane for subsequent use;
[0027] In a 250ml reaction flask, add 3.9g (0.02mol) of the compound of general formula (2) and add 80ml of dichloromethane to stir and dissolve, then add 2.8g (0.036mol) of pyridine, add dropwise the dichloromethane solution of the yellow oil, and control The temperature is below 0°C, the dropwise addition is completed, at 25°C, stir for 4 hours, stop stirring, pour the reaction solution into 120ml of ice water, add dilute hydrochloric acid under stirring, ...
Embodiment 2
[0030] Add 6.4 g (0.0238 mol) of general formula (3) into 40 ml of thionyl chloride and stir, raise the temperature and keep at 80 ° C, the reaction solids dissolve, react for 4 hours, evaporate the solvent under reduced pressure, and use 20 ml of toluene to remove the residual dichloride. Chlorothionyl, to obtain a brownish-yellow oil; dissolved in 40ml of dichloromethane for subsequent use;
[0031] In a 250ml reaction flask, add 3.9g (0.02mol) of the compound of general formula (2), add 80ml of dichloromethane and stir to dissolve, then add 2.8g (0.036mol) of n-butylamine, and add dropwise the dichloromethane solution of the yellow oil , control the temperature below 0°C, the dropwise addition is completed, 25°C, stir for 4 hours, stop stirring, pour the reaction solution into 120ml of ice water, add dilute hydrochloric acid while stirring, adjust the pH to 2, separate the organic layer and the water layer Extract with 50ml of chloroform, combine the organic layers, wash tw...
Embodiment 3
[0033] Add 6.4 g (0.0238 mol) of the general formula (3) into 160 ml of thionyl chloride, keep the room temperature at 60 ° C, react for 24 hours, evaporate the solvent under reduced pressure, and use 20 ml of toluene to remove the residual thionyl chloride to obtain brown Yellow oil; dissolved in 40ml of dichloromethane for later use;
[0034]In a 250ml reaction flask, add 3.9g (0.02mol) of the compound of general formula (2), add 80ml of chloroform, ethyl acetate and stir to dissolve, then add 2.8g (0.036mol) of 1,8-diazacyclo[5,4 , 0] Undecene-7, add dropwise the dichloromethane solution of the yellow oil, control the temperature below 0°C, the dropwise addition is completed, 25°C, stir and react for 6 hours, stop stirring, pour the reaction solution into 120ml of ice water, Add dilute hydrochloric acid under stirring, adjust the pH value to 2, separate the organic layer, extract the aqueous layer with 50ml ethyl acetate, combine the organic layer, wash twice with 100ml wat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com