Composition comprising an oligonucleotide mixture for improved detection of human papillomavirus genotypes
An oligonucleotide and composition technology, applied in the field of diagnosing different HPV genotypes, can solve problems such as inferior HPV detection, no genotyping, and reduced HPV analysis sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] HPV Sequence Alignment and HPV Primer Design
[0090] About 50 fully sequenced HPV genotypes (ie, high-risk HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52) were obtained from the National Center for Biotechnology Information (NCBI) Nucleotide Sequence Database (GenBank) , 56, 58, 59, 68, 73, 82; putative HR HPV 26, 53, and 66; and LR HPV 6, 7, 11, 13, 30, 32, 34, 40, 42, 43, 44, 54, 55 , 61, 62c, 64, 67, 69, 70, 72, 74, 81, 83, 84, 85c, 86c, 87c, 90, 97, 102, 106) L1 region nucleotide sequence, and use T-COFFEE ( Notredame, C., D.G. Higgins and J. Heringa. 2000. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J Mol Biol 302: 205-17) for comparison. BSGP5+ / 6+ primer design criteria are as follows: Prefer oligonucleotide pools rather than adding degenerate base sites in primer sequences to avoid synthetic differences in primer sequences. All primers target the same region as GP5+ / 6+. Any HPV type should: I) have no more than 3 mismatches with ...
Embodiment 2
[0103] Development of novel b-globin primers for amplification of endogenous control polynucleotides
[0104] 20 to 22 nucleotides were designed by Primer3 (Rozen et al., 2000. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132:365-86) using the b-globin gene (AY260740) as a reference sequence Long different sets of b-globin primers (Tm: 45-50°C) to amplify fragments 200 to 240 nucleotides long.
[0105] An internal DNA quality control for GP5+ / 6+ PCR has not been described. In contrast, different external b-globin PCRs act to control DNA integrity, but cannot thereby control the performance of HPV PCR and the presence and integrity of DNA in the HPV PCR reaction itself. To compensate for this limitation, new b-globin primers were incorporated in BSGP5+ / 6+ PCR as well as standard GP5+ / 6+ PCR.
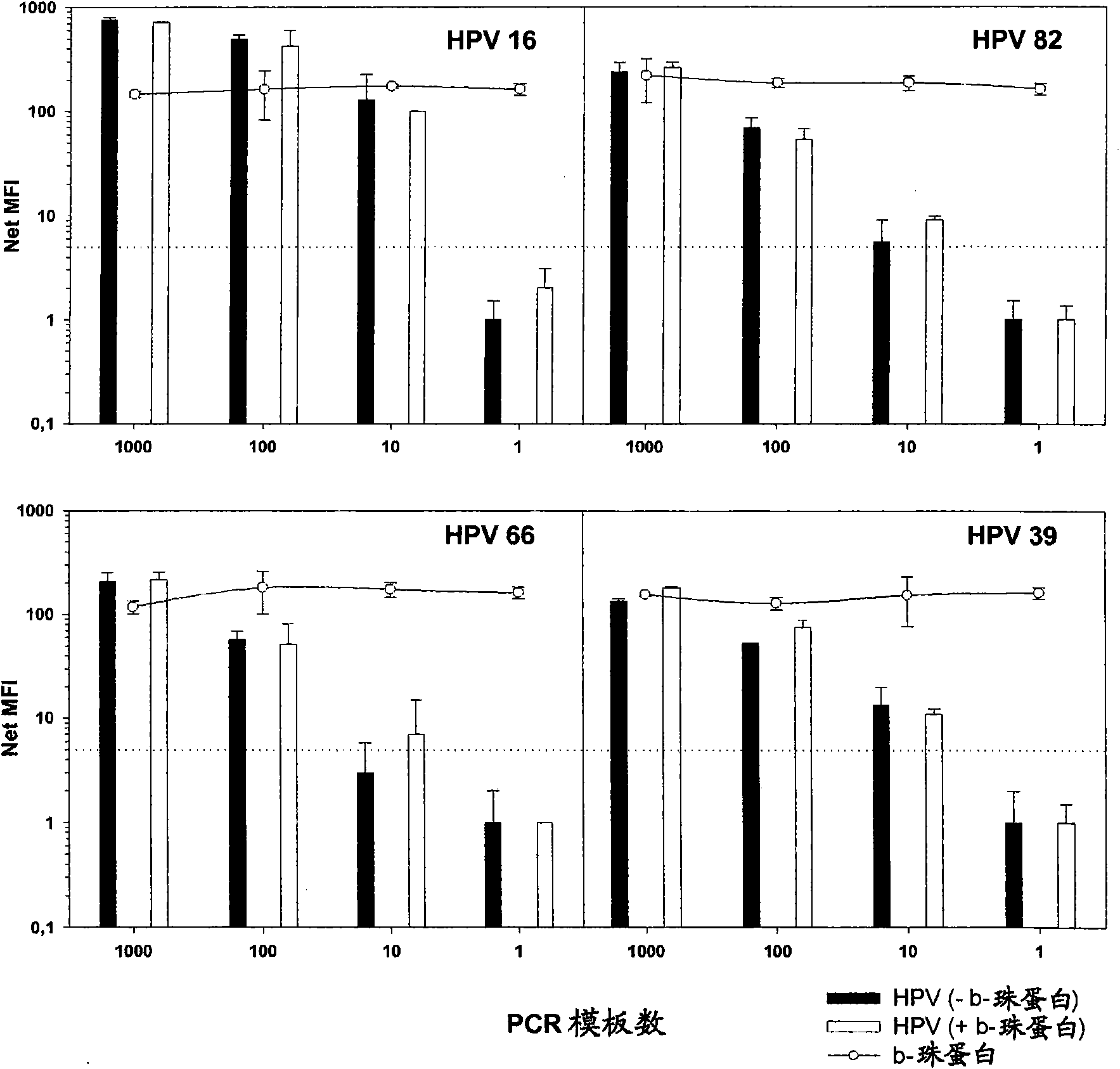
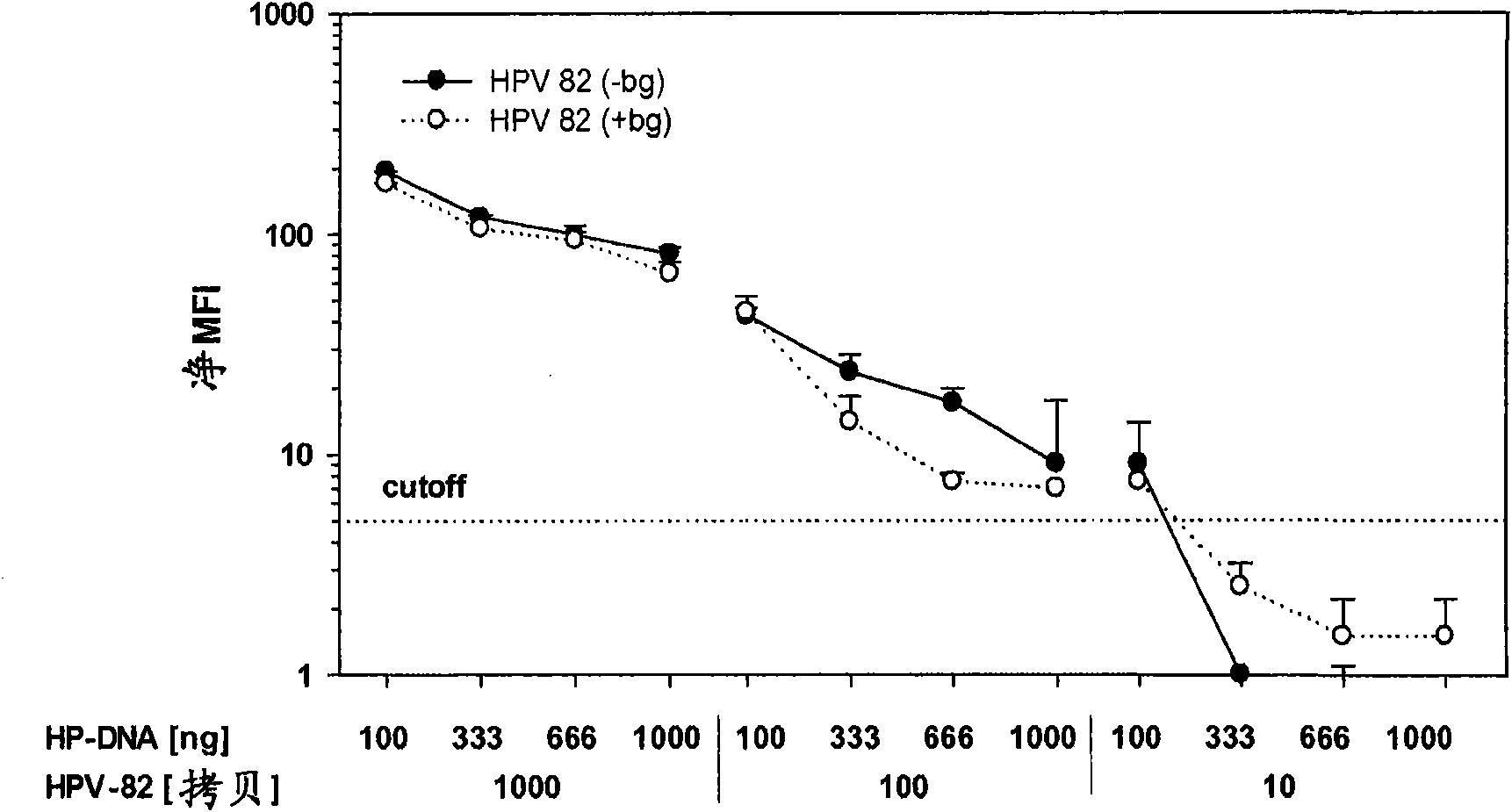
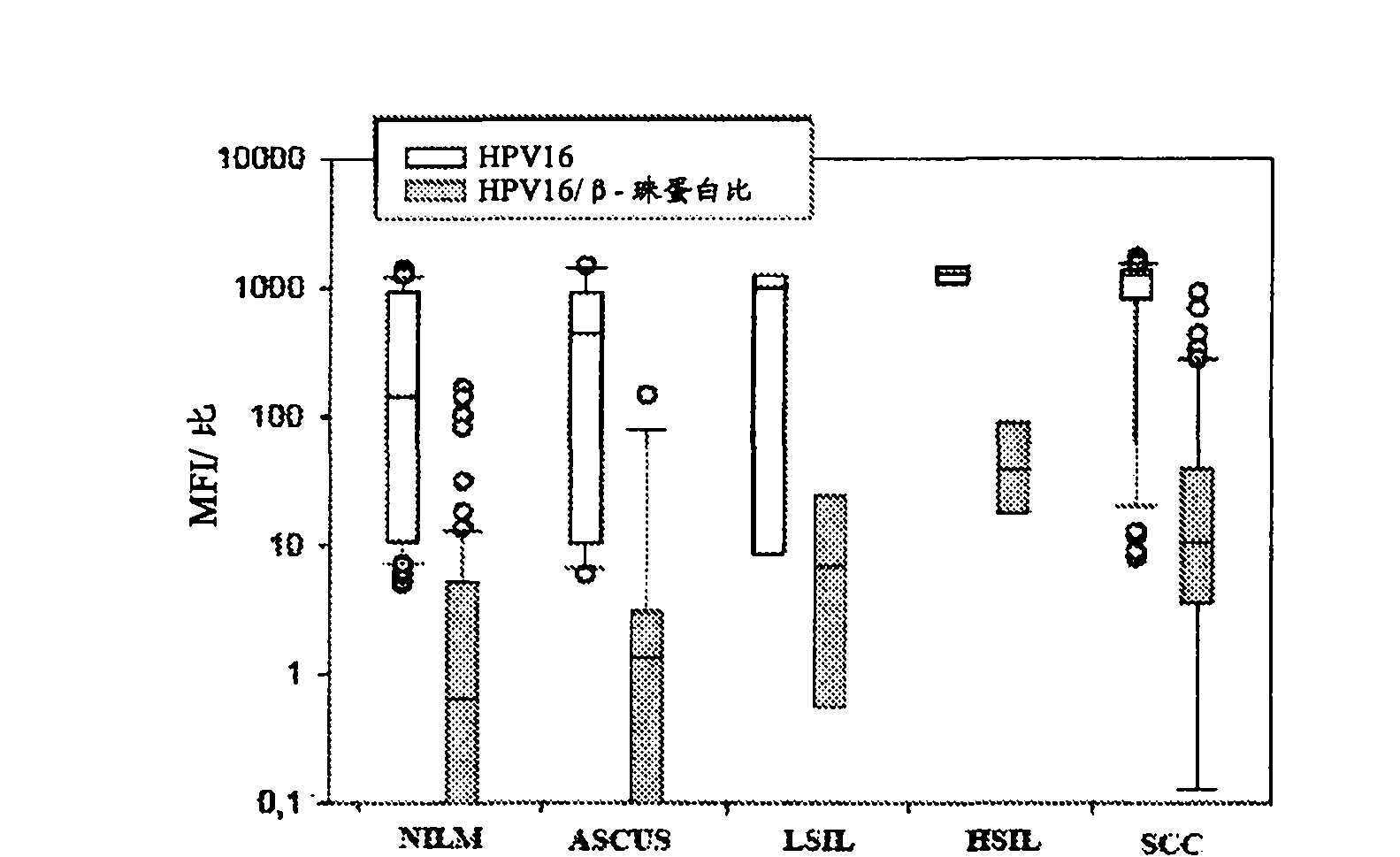
[0106] To assess the ability of GP5+ / 6+ or BSGP5+ / 6+ PCR to co-amplify DNA quality control b-globin against 10-fold dilutions containing HPV 16, ...
Embodiment 3
[0111] Amplification of cloned HPV DNA by GP5+ / 6+ and BSGP5+ / 6+ PCR
[0112] GP5+ / 6+PCR as previously described in WO 95 / 22626 and de Roda Husman, A.M., J.M. Walboomers, A.J. van den Brule, C.J.Meijer and P.J.Snijders.1995 The use of general primers GP5 and GP6 elongated at their 3′ends with adjacent highly conserved sequences Improves human papillomavirus detection by PCR. J Gen Virol 76 (Pt 4): 1057-62 as described with minor modifications. Briefly, 10 μL of DNA extracted from cervical scrapings or 1 μL of HPV plasmid dilution was diluted in 50 mM KCl, 0.8 g / L NonidetTM P40, 10 mM Tris HCl, pH 8.8 (10×PCR buffer, MBI Fermentas GmbH, St. Leon Roth, Germany), 200 μM various deoxynucleoside triphosphates (dNTPs), 3.5 mM MgCl 2(Biozym Scientific GmbH, HessischOldendorf, Germany), 1 U DNA AmpliTaq polymerase (Roche Applied Biosystems, Mannheim, Germany) and 500 nM of various GP5+ and 5' biotinylated GP6+ primers (MWG-Biotech AG, Ebersberg, Germany) in 50 μL amplified in. In th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com