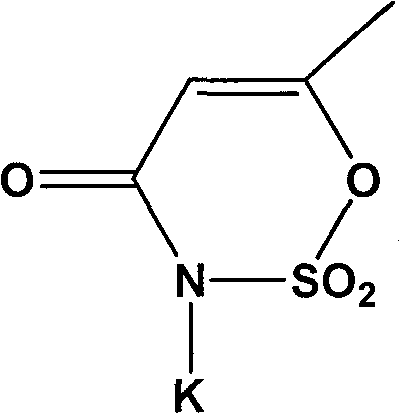
Synthesis process of acesulfame potassium
A technology for the synthesis of acesulfame potassium and its synthesis technology, which is applied in the field of synthesis of acesulfame potassium, and can solve problems such as high cost, low yield of AK sugar, and reduced product quality, and achieve good product quality, simple process, and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Take 5.0 g (50 mmol) of acetoacetamide, add 50 ml of chloroform and 50 mg of 4-N, N-dimethylaminopyridine (DMAP), stir vigorously, cool down with an ice-salt bath, and control the temperature at -30 to -25 ° C, slowly Add a solution of 10ml of chlorosulfonic acid and 10ml of chloroform, keep stirring at low temperature for 1.0 hours after the addition, raise the temperature to -10°C, slowly add 8ml of water, then stir for 45 minutes, let stand to separate layers, add 20ml to the water layer Extract with chloroform, remove the water phase, combine the organic phases and add 50ml of water, neutralize with 10% potassium hydroxide to pH=7.5, let stand to separate layers, recover the solvent chloroform, spin dry the water under reduced pressure to obtain the crude product of acesulfame potassium 7.2 g was dissolved in an appropriate amount of water and heated to a slight boil, 50 mg of activated carbon was added to reflux for 10 minutes, and 6.5 g of the product was obtained ...
Embodiment 2
[0027] Take 5.0g (50mmol) of acetoacetamide, add 50ml of dichloromethane and 50mg of 4-N,N-dimethylaminopyridine (DMAP), stir vigorously, cool down with an ice-salt bath, and control the temperature at -30~-25°C , slowly add a solution of 10ml of chlorosulfonic acid and 10ml of dichloromethane, keep stirring at low temperature for 1.0 hour after the addition, raise the temperature to -10°C and slowly add 8ml of water, then stir for 45 minutes, let stand to separate layers, and Add 20ml of chloroform to the aqueous layer for extraction, remove the aqueous phase, combine the organic phases and add 50ml of water, neutralize with 10% potassium hydroxide to pH = 7.5, let stand to separate layers, recover the solvent chloroform, spin dry under reduced pressure to obtain crude acetyl 7.3 g of crude potassium sulfamate was dissolved in an appropriate amount of water and heated to a slight boil, 50 mg of activated carbon was added to reflux for 10 minutes, and 6.6 g of the product was o...
Embodiment 3
[0029] Take 5.0g (50mmol) of acetoacetamide, add 50ml of dichloroethane and 50mg of 4-N,N-dimethylaminopyridine (DMAP) and stir vigorously, cool down with an ice-salt bath, and control the temperature at -30~-25°C , slowly add a solution of 10ml of chlorosulfonic acid and 10ml of dichloroethane, keep stirring at low temperature for 1.0 hour after the addition is complete, raise the temperature to -10°C, slowly add 8ml of water, then stir for 45 minutes, let stand to separate layers, Add 20ml of chloroform to the aqueous layer for extraction, remove the aqueous phase, combine the organic phases and add 50ml of water, neutralize with 10% potassium hydroxide to pH = 7.5, let stand to separate layers, recover the solvent chloroform, spin dry the water under reduced pressure to obtain the crude product 7.2 g of crude acesulfame potassium was dissolved in an appropriate amount of water and heated to a slight boil, 50 mg of activated carbon was added to reflux for 10 minutes, and 6.5 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com