Salt compound of tenofovir disoproxil fumarate and preparation method and medicinal application thereof
A technology of tenofovir disoproxil and phosphoric acid, which is applied in the field of medicine and can solve problems such as differences in bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1. New crystal form of tenofovir disoproxil phosphate and its preparation
[0036] In a 50ml reaction bottle, add 2.0g of tenofovir disoproxil and 20.0ml of isopropanol, heat to 50°C, stir, add phosphoric acid dropwise to the solution pH2-3, drop it, react for 30min, cool to 0°C and place for 8hr, Suction filtration to obtain a white crystalline powder, washed twice with a small amount of isopropanol, and vacuum-dried to obtain the new crystal form of tenofovir disoproxil phosphate of the present invention.
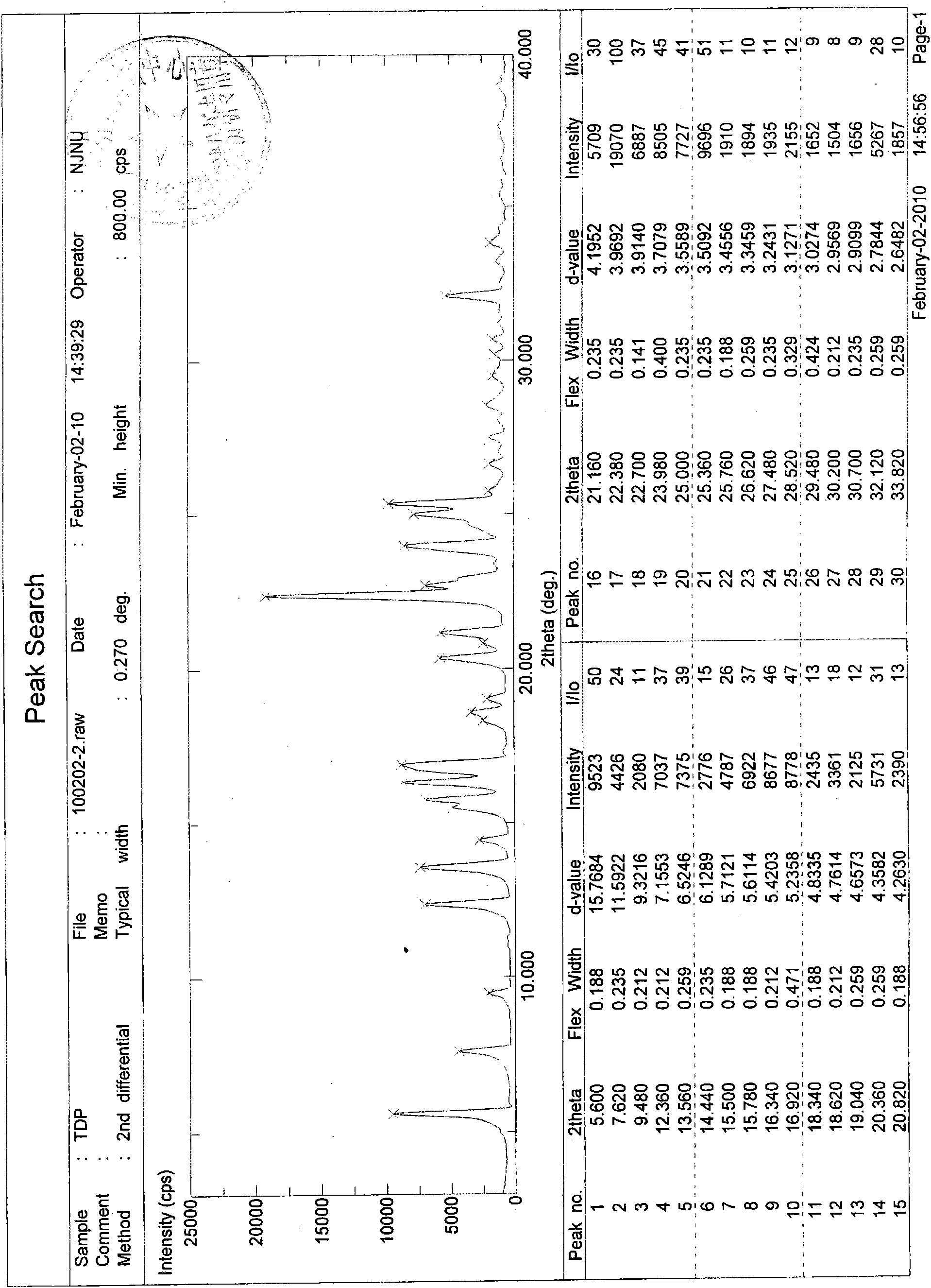
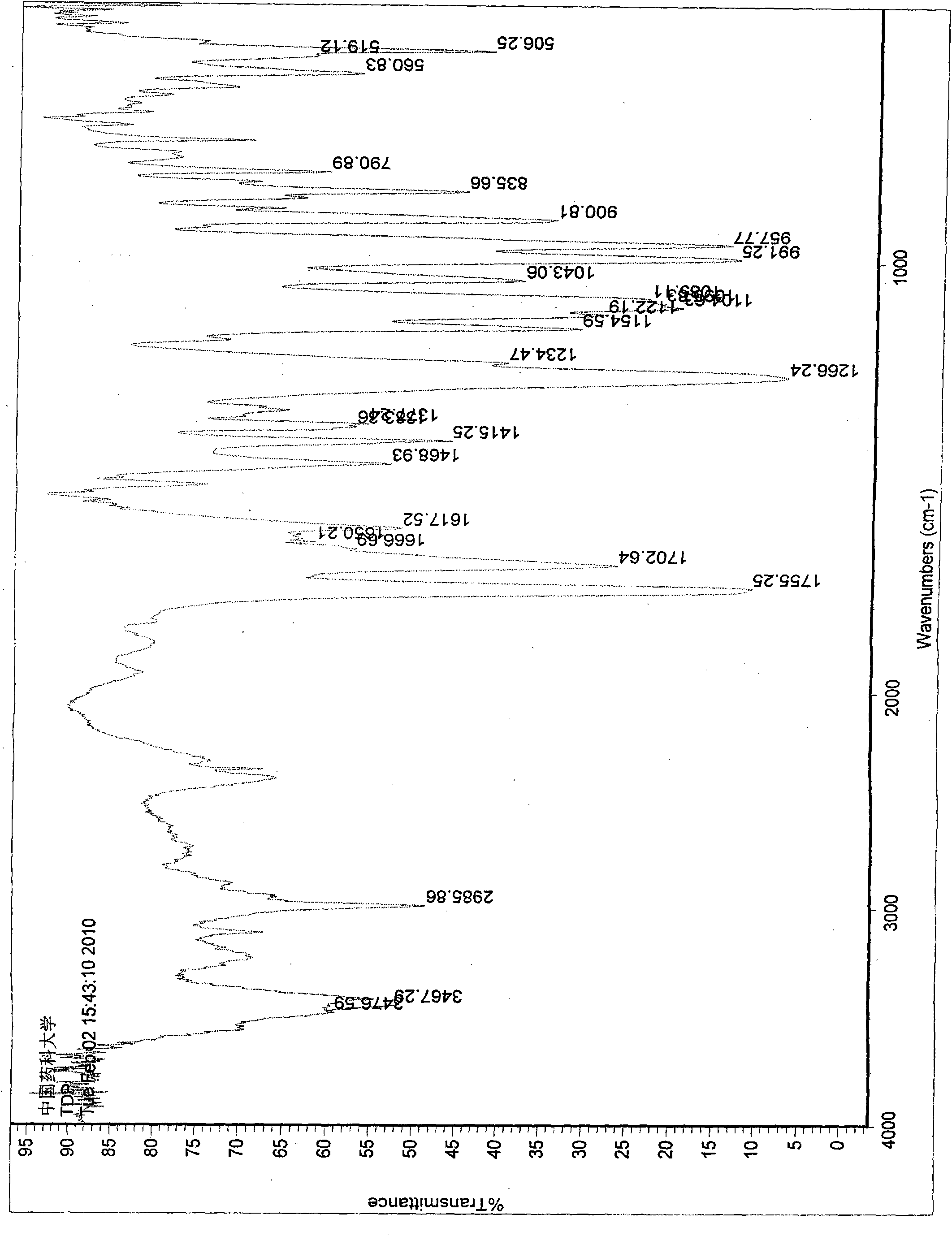
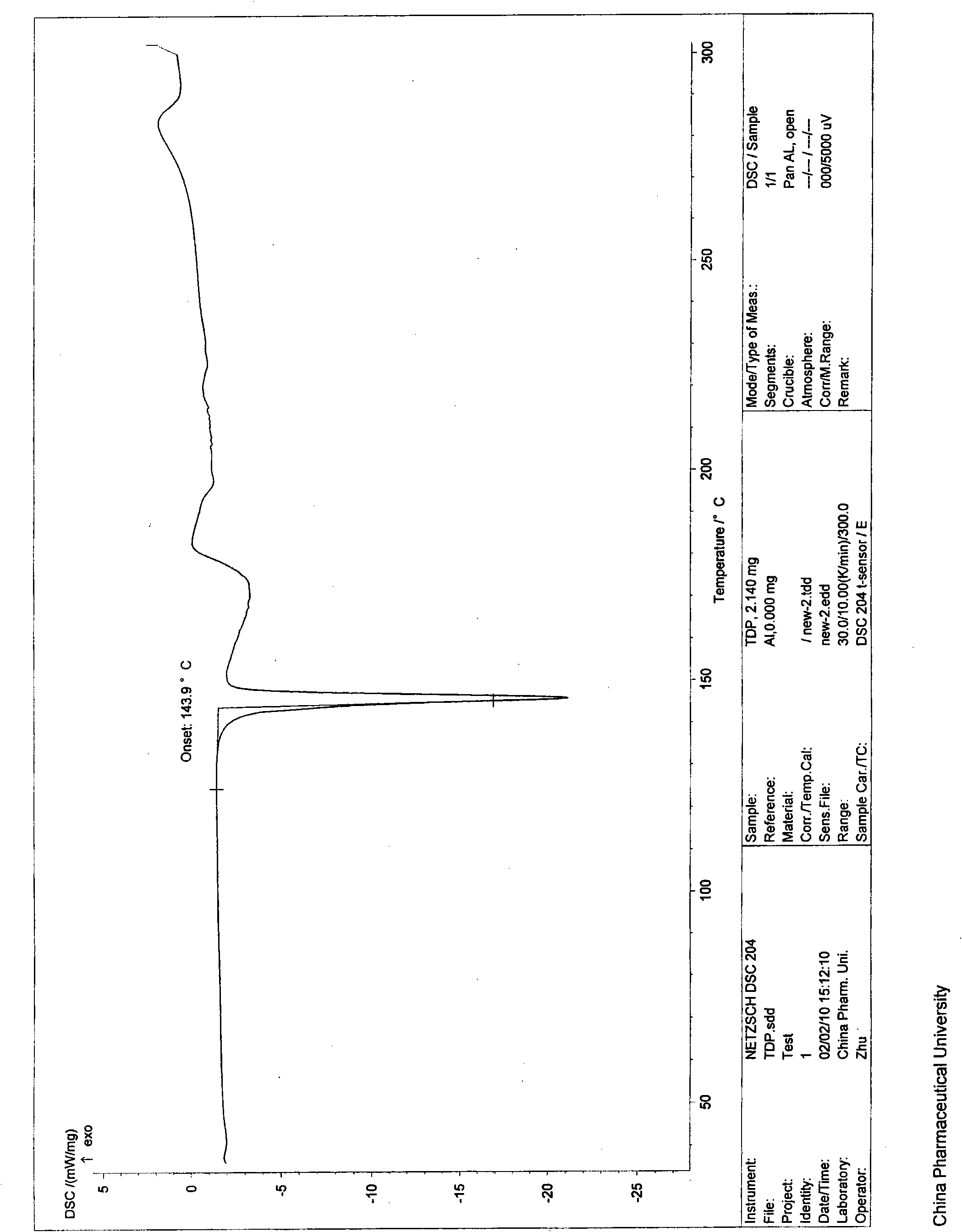
[0037] Its X-ray diffraction diagram, infrared absorption spectrum diagram and differential thermal analysis diagram are shown in the appendix figure 1 , 2 and 3.
Embodiment 2
[0038] Embodiment 2. tenofovir disoproxil phosphate tablet
[0039] Prescription: 300 g of the new crystal form of tenofovir disoproxil phosphate of the present invention, 100 g of hydroxypropyl cellulose, and 50 g of sodium carboxymethyl starch.
[0040] Preparation method: The above-mentioned two kinds of materials were crushed through 100 mesh respectively, dried under reduced pressure at 100°C for more than 10 hours, cooled to room temperature, mixed with the new crystal form of tenofovir disoproxil phosphate, granulated by a dry granulator, and pressed into 1000 tablets, that is.
Embodiment 3
[0041] Example 3. Tenofovir Disoproxil Phosphate Capsules
[0042] Prescription: 300g of the new crystal form of tenofovir disoproxil phosphate of the present invention, 65g of lactose, 70g of microcrystalline cellulose, 15g of sodium carboxymethyl starch, and 1.5g of magnesium stearate.
[0043] Preparation method: the above-mentioned lactose, microcrystalline cellulose, and sodium carboxymethyl starch were respectively crushed through 100 meshes, dried under reduced pressure at 100°C for more than 10 hours, cooled to room temperature, and mixed evenly with the new crystal form of tenofovir disoproxil phosphate , granulated by a dry granulator, added magnesium stearate, mixed evenly, filled into 1000 empty capsules, and obtained tenofovir disoproxil phosphate capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com