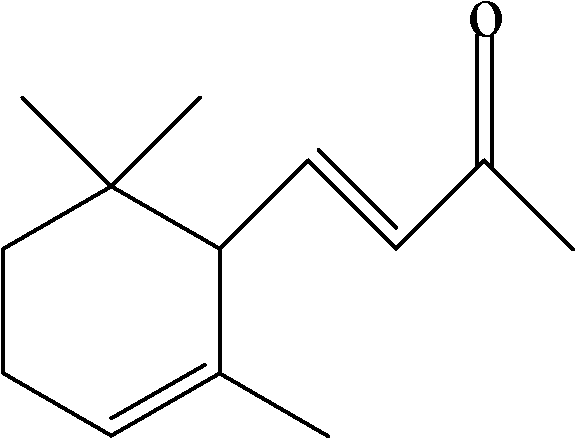
Method for synthesizing 4,5-epoxy-alpha-ionone
A technology of ionone and synthesis method, which is applied in 4 fields, can solve the problems of many by-products, difficult recovery, low product selectivity, etc., and achieve the effect of simple process and no pollution to the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 20.0ml of α-ionone, 10.0ml of chloroform and 0.50g of Au / MCM-41 molecular sieve catalyst into the autoclave, feed 1.0Mpa oxygen, react at 60°C for 12h, and detect the reaction solution by GC-MS chromatography, α - The conversion of ionone is 98%, the selectivity of 4,5-epoxy-α-ionone is 75%.
Embodiment 2-4
[0018] According to the method and steps of Example 1, but the solvent used is ethanol, acetonitrile, acetone. The results are respectively: the conversion rate of α-ionone is 75%, the selectivity of 4,5-epoxy-α-ionone is 60%; the conversion rate of α-ionone is 91%, 4,5-epoxy The selectivity of -α-ionone was 63%; the conversion rate of α-ionone was 95%, and the selectivity of 4,5-epoxy-α-ionone was 70%.
Embodiment 5
[0020] According to the method and steps of Example 1, but the reaction time is 8h. The results were: the conversion rate of α-ionone was 81%, and the selectivity of 4,5-epoxy-α-ionone was 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com