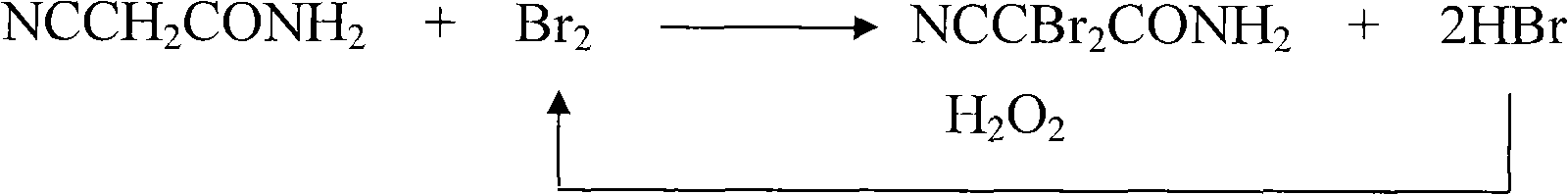
Synthesis method of 2,2-dibromo-2-malonamidenitrile
A technology of cyanoacetamide and cyanoacetamide, which is applied in 2 fields, can solve problems such as easy product decomposition, high reaction temperature, and low reaction yield, and achieve stable yield, improved reaction yield, and small fluctuation range Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 84.1g (1.0mol) of cyanoacetamide and 252mL of water into a four-necked reaction flask, and add 192.7g (99.5%, 1.2mol) of liquid bromine dropwise under stirring at a reaction temperature of 20°C to 25°C. When the amount of addition is 2 / 3 of the total amount, start to add 80.7mL (30.5%, 0.8mol) of hydrogen peroxide dropwise. After the liquid bromine and hydrogen peroxide are added dropwise, continue the constant temperature reaction for 1.5h, filter, and recover the filtrate. It is the product 2,2-dibromo-2-cyanoacetamide with a yield of 99.6%. The experiment was repeated four times under the same process conditions, and the reaction yields were 99.0%, 99.5%, 98.7%, and 99.3%, respectively, and the fluctuation range of the reaction yield was less than 1%.
Embodiment 2
[0023] Add 84.1g (1.0mol) of cyanoacetamide and 336mL of water into a four-necked reaction flask, and add 208.8g (99.5%, 1.3mol) of liquid bromine dropwise under stirring at a reaction temperature of 15°C to 20°C. When the amount added is 1 / 2 of the total amount, start to add 70.6mL (30.5%, 0.7mol) of hydrogen peroxide dropwise. After the liquid bromine and hydrogen peroxide are added dropwise, continue the constant temperature reaction for 0.5h, filter, and recover the filtrate. It is the product 2,2-dibromo-2-cyanoacetamide with a yield of 98.4%.
Embodiment 3
[0025] Add 84.1g (1.0mol) of cyanoacetamide and 420mL of water into the four-necked reaction flask, and add 176.7g (99.5%, 1.1mol) of liquid bromine dropwise under stirring at a reaction temperature of 40°C to 45°C. When the addition amount is 1 / 3 of the total amount added, start to add 90.8mL (30.5%, 0.9mol) of hydrogen peroxide dropwise. After liquid bromine and hydrogen peroxide are added dropwise, continue the constant temperature reaction for 4h, filter, and recover the filtrate. The filter cake is Product 2, 2-dibromo-2-cyanoacetamide, yield 82.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com