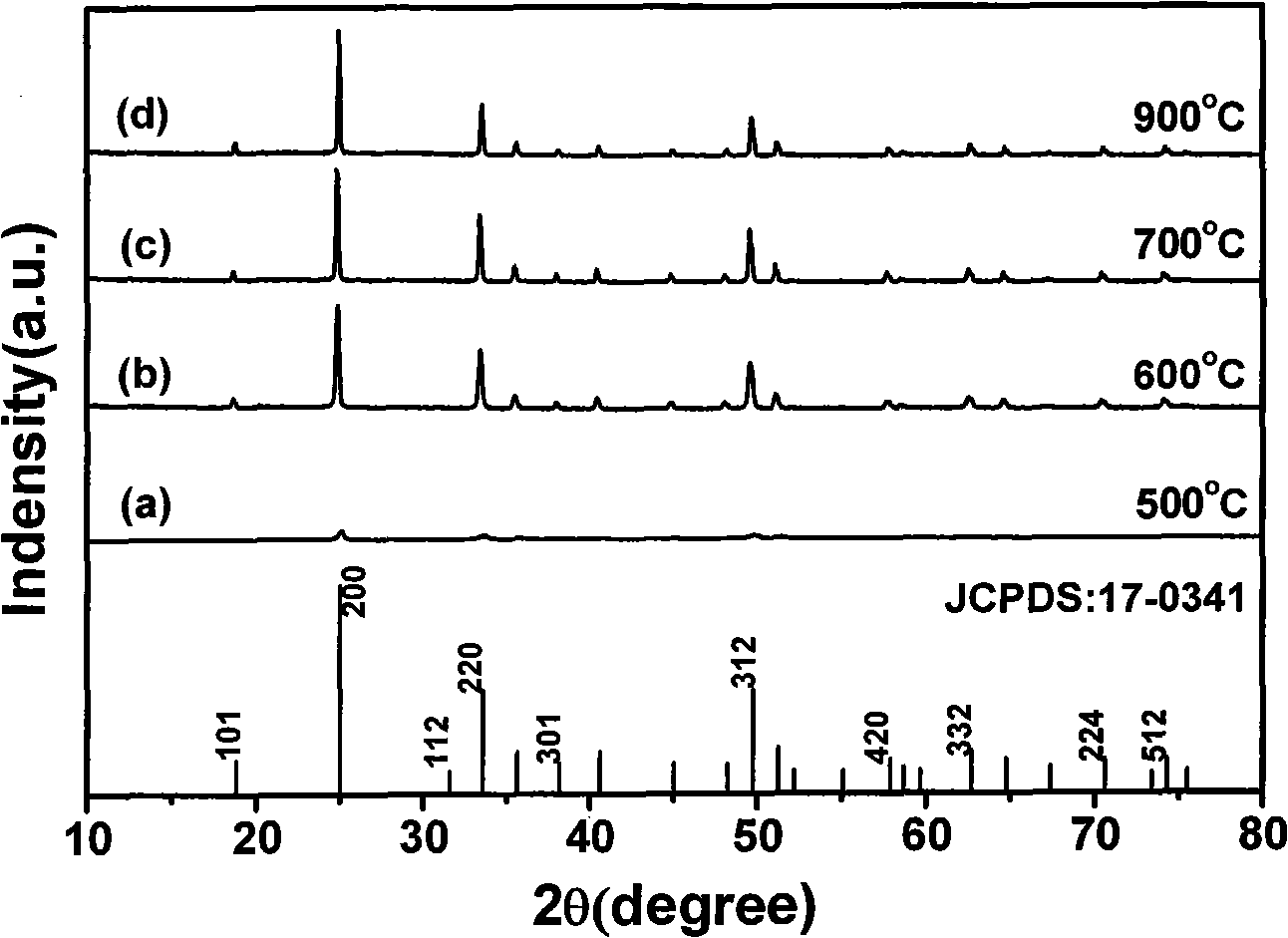
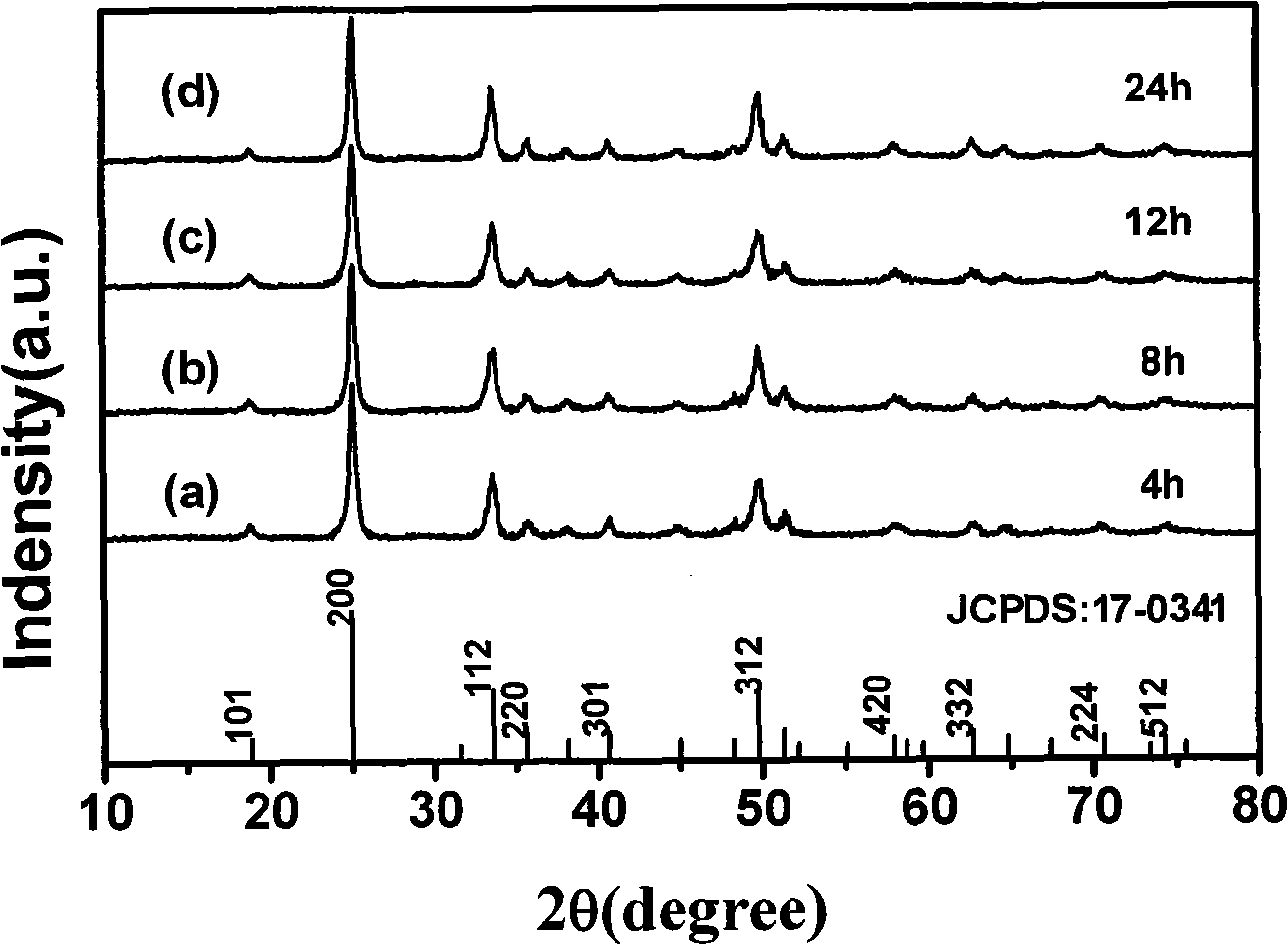
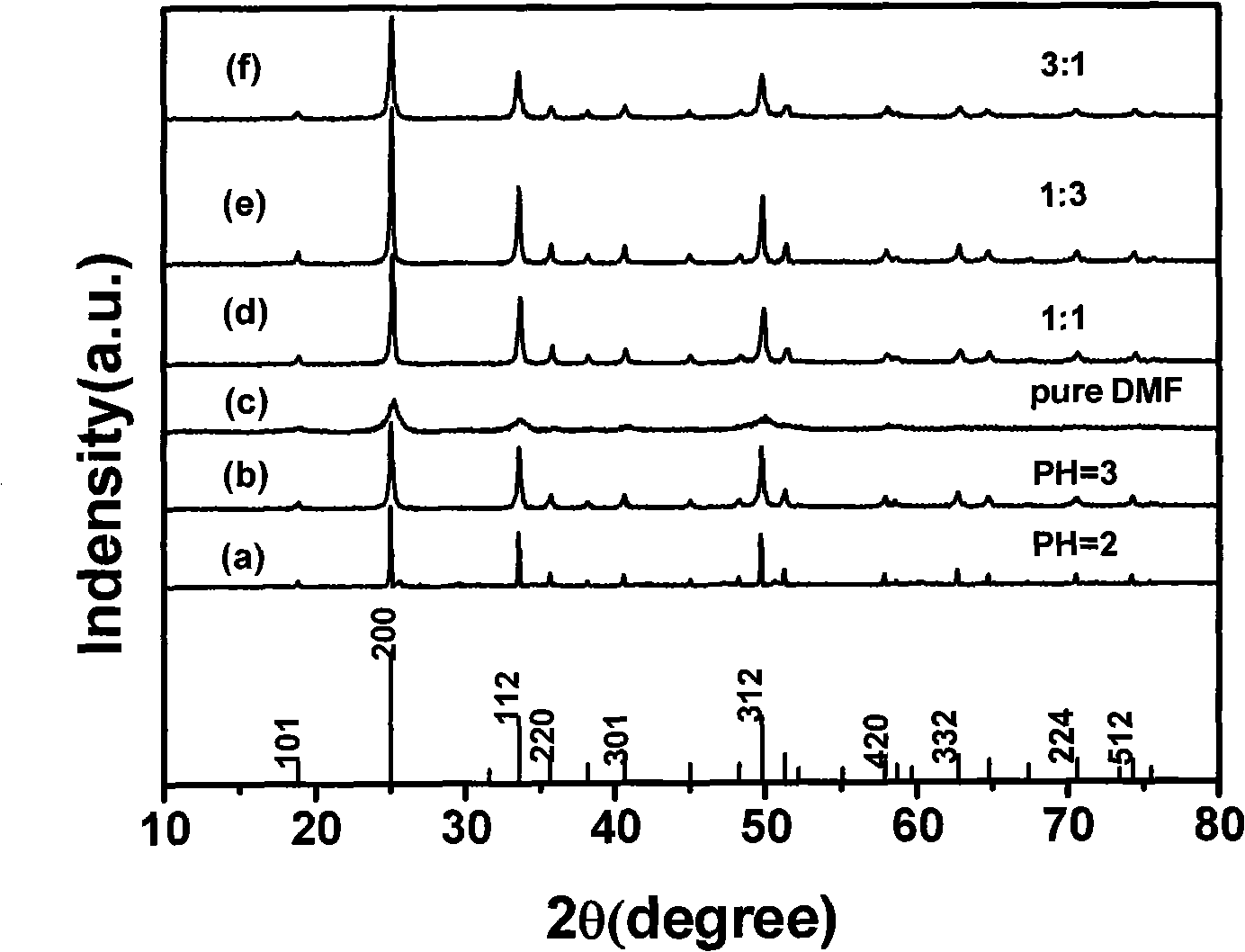
Method for preparing yttrium vanadate crystals
A technology of yttrium vanadate and crystal, applied in the field of preparation of yttrium vanadate crystal, can solve the problems of high sintering temperature, long synthesis time, difficult to obtain products, etc., and achieve the effect of high luminous intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The starting material used is N, N-dimethylformamide (DMF) HCON (CH 3 ) 2 (analytical grade), ammonium metavanadate NH 4 VO 3 (analytical pure), europium trioxide Eu 2 o 3 (99.99%), diyttrium trioxide Y 2 o 3 (analytical pure), concentrated nitric acid HNO 3 (analytical grade), polyvinylpyrrolidone (PVP) (C 6 h 9 NO) n (analytical grade), ethylene glycol HOCH 2 CH 2 OH (analytical pure).
[0033] Weigh 0.117g (0.1mmol) NH 4 VO 3 Solid dissolved in 1mL HNO 3 In, N,N-dimethylformamide and water were added thereto to maintain a volume ratio of 3:1, and 0.4 g of PVP was added. Weigh 0.107gY 2 o 3 Put in a beaker, add 2mLHNO 3 and 2mLH 2 O, heated to a certain temperature to a high-concentration molten state, and then added 3mLH 2 O, so repeated 2 times, then immediately add the solution obtained in the previous step and stir for 20min. Add 1 mL of Eu(NO 3 ) 3 , and then stirred for 30min. The mixture was transferred to a reaction kettle lined with po...
Embodiment 2
[0035] Weigh 0.117g (0.1mmol) NH 4 VO 3 Solid dissolved in 1mL HNO 3 In, N,N-dimethylformamide and water were added thereto to maintain a volume ratio of 1:1, and 0.4 g of PVP was added. Weigh 0.107gY 2 o 3 Put in a beaker, add 2mLHNO 3 and 2mLH 2 O, heated to a certain temperature to a high-concentration molten state, and then added 3mLH 2 O, so repeated 2 times, then immediately add the solution obtained in the previous step and stir for 20min. Add 1 mL of Eu(NO 3 ) 3 , and then stirred for 30min. The mixture was transferred to a reaction kettle lined with polytetrafluoroethylene, sealed, and reacted at 180°C for 24h. After the reactor was naturally cooled to room temperature, the resulting precipitate was centrifuged, washed repeatedly with ethanol and distilled water, dried at 80°C for 12 hours, and then the sample was heated at room temperature at 1°C / min to 600°C for 3 hours at this temperature.
Embodiment 3
[0037] Weigh 0.117g (0.1mmol) NH 4 VO 3 Solid dissolved in 1mL HNO 3 In, N,N-dimethylformamide and water were added thereto to maintain the volume ratio of 1:3, and 0.4 g of PVP was added. Weigh 0.107gY 2 o 3 Put in a beaker, add 2mLHNO 3 and 2mLH 2 O, heated to a certain temperature to a high-concentration molten state, and then added 3mLH 2 O, so repeated 2 times, then immediately add the solution obtained in the previous step and stir for 20min. Add 1 mL of Eu(NO 3 ) 3 , and then stirred for 30min. The mixture was transferred to a reaction kettle lined with polytetrafluoroethylene, sealed, and reacted at 180°C for 24h. After the reactor was naturally cooled to room temperature, the resulting precipitate was centrifuged, washed repeatedly with ethanol and distilled water, dried at 80°C for 12 hours, and then the sample was heated at room temperature at 1°C / min to 600°C for 3 hours at this temperature.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com