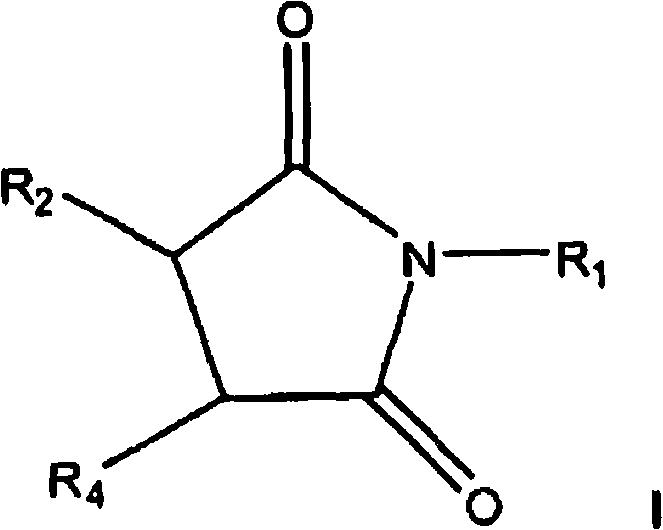
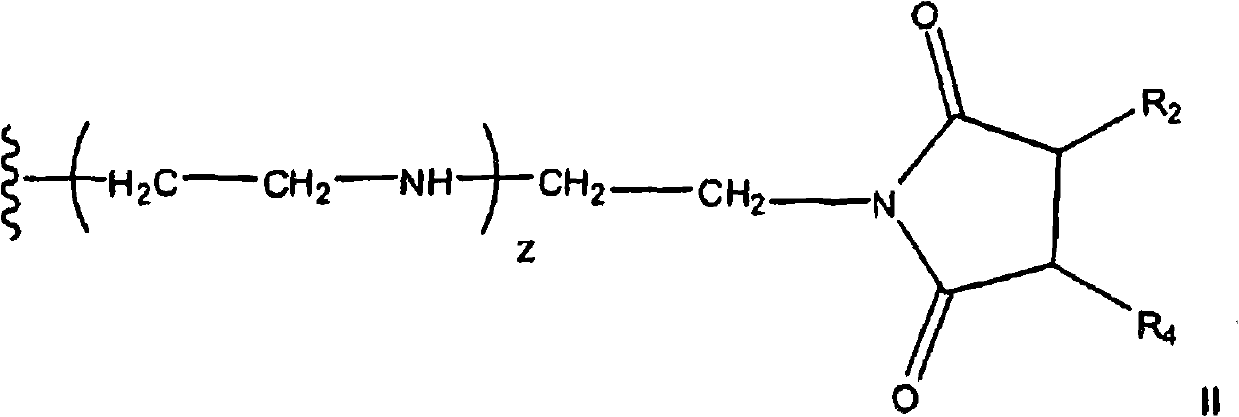
Pyrrolidine-2,5-dione derivatives for use in friction modification
A technology of tribology and alkyl, applied in the direction of lubricating composition, organic chemistry, additives, etc., can solve problems such as no public or recognized suggestions, no solution to low-temperature viscosity problems, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Embodiment 1
[0124] 339.4 grams of C containing about forty (40) wt.% inner vinylidene 20 -C 24 Olefin (1.1 mol), 112.8 grams (1.2 mol) of molten maleic anhydride, 2.42 grams of p-toluenesulfonic acid (TsOH) and 1.64 grams of synthetic antioxidant (a hindered phenol antioxidant) were charged into a 1.0 L autoclave. The stirred mixture was subjected to vacuum (28 in. Hg), nitrogen purge, and vacuum cycles while stirring with heating. After about 5 hours at a temperature of about 225°C, the formed alkylsuccinic anhydride is transferred to a separate reactor where unreacted maleic anhydride is removed by distillation. Ammonia gas (16.95 grams) was bubbled into the stirred mixture at about 160°C for about 2 to about 3 hours. The hot mixture was vacuum filtered to give a clear product containing 2.29% N. The infrared spectrum of this transparent product shows that the carbonyl bond is at 1771, 1709cm -1 (imide). The amount of acid groups titratable by a strong base is 2.07 milliequivalents...
Embodiment 2
[0125] 450.0 g linear αC 20 -C 24 Olefin (1.5 mol), 149.6 g (1.5 mol) of molten maleic anhydride, 3.21 g of p-toluenesulfonic acid (TsOH) and 2.20 g of synthetic antioxidant (a hindered phenol antioxidant) were charged into a 2.0 L autoclave. The stirred mixture, sealed under vacuum, was stirred and heated at 225°C for approximately 5 hours. The resulting alkylsuccinic anhydride is transferred to a separate reactor where unreacted maleic anhydride is removed by distillation. A portion of the intermediate product (99.0 g) was neutralized with ammonia gas (4.21 g) at 160°C to yield 98.8 g of succinimide containing 2.94% N. Representative isomerized linear C 20 Succinimide structures include (E)-3-(eicosyl-8-en-8-yl)pyrrolidine-2,5-dione, shown above. Example 3
Embodiment 3
[0126] 344.9 grams of C containing about forty (40) wt.% inner vinylidene 20 -C 24 Olefin (1.1 mol), 114.6 g (1.2 mol) of molten maleic anhydride, 2.46 g of p-toluenesulfonic acid (TsOH) and 1.67 g of synthetic antioxidant (a hindered phenol antioxidant) were charged into a 1.0 L autoclave. The stirred mixture was subjected to vacuum (28 in. Hg), nitrogen purge, and vacuum cycles while stirring with heating. After about 5 hours at about 225°C, the formed alkylsuccinic anhydride is transferred to a separate reactor where unreacted maleic anhydride is removed by distillation. The product was diluted with 45.0 g of process oil. 43.9 grams of commercial tetraethylenepentamine (TEPA) was added dropwise under temperature-controlled conditions, resulting in a mixture stirred at 160°C under vacuum for 3 hours. The product (258.5 g) contained 6.09% N and had a total base number (TBN) of 146.5 mg KOH / g. Representative vinylidene C 20 Bis-succinimide structures include (2,2'-azanedi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| flash point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com