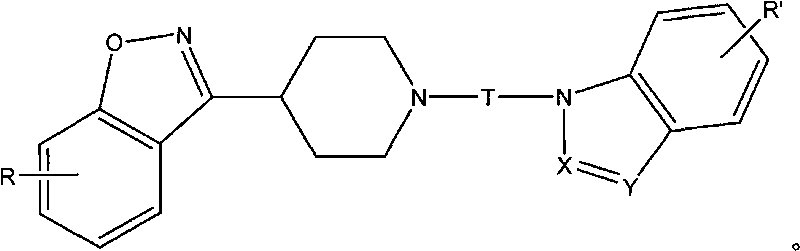
Benzo-isoxazol piperidine derivative and application in preparing analgesic and sedative medicaments
A technology of benzisoxazole piperidine and fluorobenisoxazole is applied to benzisoxazole piperidine derivatives and application fields in the preparation of analgesic and sedative drugs, and can solve the problem of poor treatment effect and inability to Meet clinical treatment requirements and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Preparation of N-(2-(1-indolyl)ethyl)-4-(3-(6-fluorobenzoisoxazolyl))piperidine (II-1) hydrochloride
[0098] Using indole as a raw material, N-(2-chloroethyl)indole was prepared according to the synthesis and post-treatment methods in General Method 1. Take N-(2-chloroethyl)indole (1.0g, 0.0055mol), 4-(3-(6-fluorobenzoisoxazolyl))piperidine (1.10g, 0.005mol), DIPEA (2.58 g, 0.02mol) and KI (0.83g, 0.005mol) in 30ml of acetonitrile solution, reflux reaction for 12 hours, according to the post-treatment operation in General Method 1, 1.31g of white crystals were obtained with a yield of 65.5%. Melting point: 216-218°C.
[0099] Elemental Analysis: C 22 h 22 FN 3 O·HCl·H 2 O (Theoretical %: C 63.23, H 6.03, N 10.05, Cl 8.48; Experimental % C 63.15, H 6.021, N 10.08, Cl 8.51); MS: m / z 363.2 (M + )
[0100] 1 HNMR (DMSO-d 6 ): δ2.21~2.25(m, 2H), 2.34~2.44(m, 2H), 3.13~3.22(m, 2H,), 3.43~3.52(m, 3H), 3.63~3.67(m, 2H), 4.76 ~4.81(m, 2H), 6.50~6.52(d, 1H, J=3.2 Hz,),...
Embodiment 2
[0102] Preparation of N-(3-(1-indolyl)propyl)-4-(3-(6-fluorobenzoisoxazolyl))piperidine (II-2) hydrochloride
[0103] Using indole as raw material, N-(3-chloropropyl)indole was prepared according to the synthesis and post-treatment methods in General Method 1. Take N-(3-chloropropyl)indole (1.07g, 0.0055mol), 4-(3-(6-fluorobenzoisoxazolyl))piperidine (1.10g, 0.005mol), DIPEA (2.58 g, 0.02mol) and KI (0.83g, 0.005mol) in 30ml of acetonitrile solution, reflux reaction for 12 hours, according to the post-treatment operation in General Method 1, 1.28g of white crystals were obtained with a yield of 61.8%. Melting point: 209-211°C.
[0104] Elemental Analysis: C 23 h 24 FN 3 O·HCl·2H 2 O (Theoretical %: C 66.74, H 6.02, N 10.15, Cl 8.57; Experimental % C 66.70, H 6.01, N 10.12, Cl 8.55); MS: m / z 377.2 (M + )
[0105] 1 HNMR (DMSO-d 6 ): δ2.12~2.20(m, 2H), 2.21~2.25(m, 2H), 2.34~2.45(m, 2H), 3.14~3.22(m, 2H), 3.42~3.50(m, 3H)3.64~ 3.67(m, 2H), 4.77~4.80(m, 2H 2 ), 6.52-8....
Embodiment 3
[0107] Preparation of N-(4-(1-indolyl)butyl)-4-(3-(6-fluorobenzoisoxazole))piperidine (II-3) hydrochloride
[0108] Using indole as raw material, N-(4-chlorobutyl)indole was prepared according to the synthesis and post-treatment methods in General Method 1. Take N-(4-chlorobutyl)indole (1.14g, 0.0055mol), 4-(3-(6-fluorobenzoisoxazolyl))piperidine (1.10g, 0.005mol), DIPEA (2.58 g, 0.02mol) and KI (0.83g, 0.005mol) in 30ml of acetonitrile solution, reflux reaction for 16 hours, according to the post-treatment operation in General Method 1, 1.33g of white crystals were obtained, with a yield of 62.1%. Melting point: 201-203°C.
[0109] MS: m / z 391.2 (M + )
[0110] 1 HNMR (DMSO-d 6 ): 1.68~1.74(m, 2H), 1.79~1.85(m, 2H), 2.16~2.21(m, 2H), 2.27~2.37(m, 2H), 3.00~3.13(m, 4H), 3.41~3.48 (m, 1H), 3.53~3.57(m, 2H), 4.20~4.25(t, 2H, J=6.8Hz), 6.43(d, 1H, J=6.4Hz), 7.01(t, 1H, J=7.6 Hz), 7.13(t, 1H, J=7.6Hz), 7.33(td, 1H, J=9.2Hz, J=2.0Hz), 7.42(d, 1H, J=3.2Hz), 7.52(d, 1H, J=7.6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com