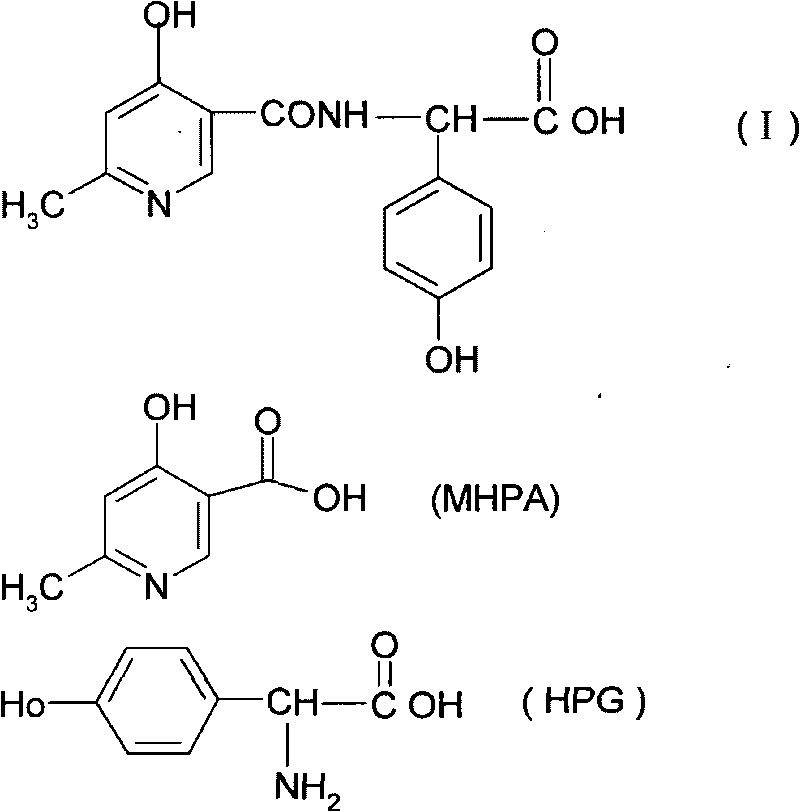
Composite method for cefpiramide midbody D-alpha-(4-Hydroxy-6-methylnicotinamido) hydroxyphenylacetic acid
A technology of hydroxynicotinamide group and p-hydroxyphenyl group, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of cumbersome operation and a large amount of waste water, and achieves the effects of small waste water volume, easy treatment and low comprehensive cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
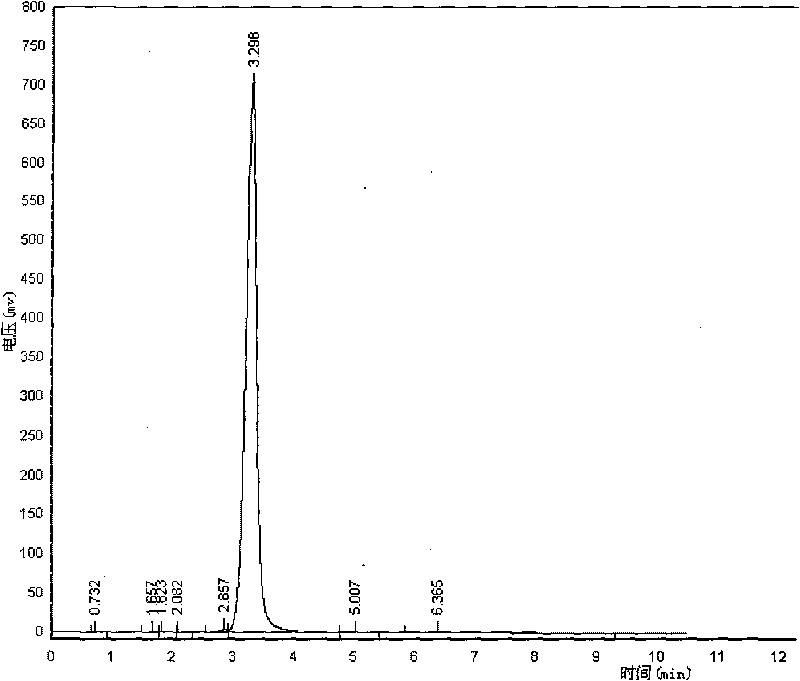
[0038] Embodiment 1: synthetic reaction route is as follows:
[0039]
[0040] (1) Suspend 13.36g of HPG in 120ml of dichloromethane as an organic solvent, cool to 10°C, then add 27g of trimethylchlorosilane (silicon esterifying agent) at one time, and then slowly add organic solvent at 15°C to 20°C Base 24.24g of triethylamine, keep stirring at the same temperature for 1.5 hours to obtain HPG silicon esterification solution.
[0041] (2) Take 15g of MHPA and suspend it in 140ml of methylene chloride, and use the MHPA acyl chloride prepared by the conventional acyl chloride preparation method.
[0042] (3) Control the temperature at 15°C to 18°C, slowly add MHPA acid chloride dropwise to the HPG silicon esterification solution in step (1), keep the same temperature for 1.5 hours, then add 260ml deionized water, and use 10% NaOH The pH of the solution was adjusted to 7.1. Still layering.
[0043](4) Decolorize the water layer with activated carbon, remove the activated ca...
Embodiment 2
[0045] As described in Example 1, the difference is that dichloroethane is used as the organic solvent, and the result is 20.3 grams of D-α-(6-methyl-4-hydroxynicotinamide) p-hydroxyphenylacetic acid, yield 84.02% .
Embodiment 3
[0047] As described in Example 1, the difference is that the silicon esterification agent uses diethylmethylchlorosilane to obtain 19 grams of D-α-(6-methyl-4-hydroxynicotinamide) p-hydroxyphenylacetic acid crystals , yield 78.64%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com