Compound nateglinide valsatan medicinal composition
A technology of nateglinide and composition, applied in the field of double-layer tablet and preparation thereof, can solve the problems of inability to carry out satisfactory dissolution, poor disintegration, inability to exert quick effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
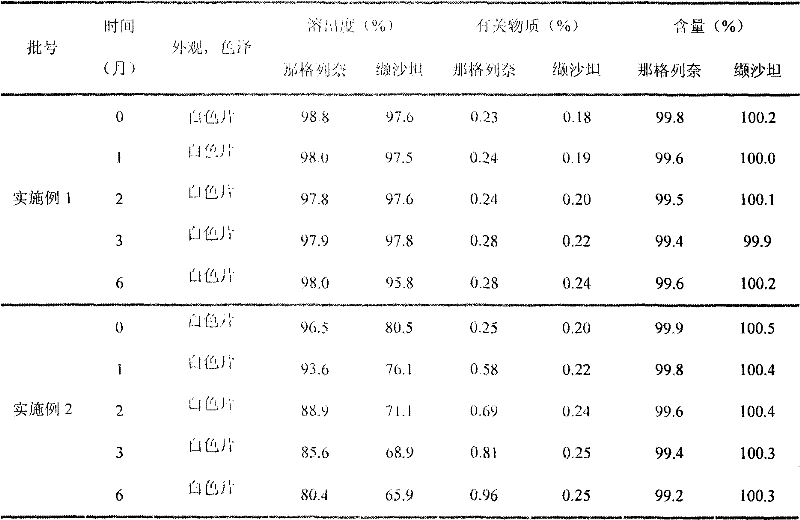
Examples
Embodiment 1
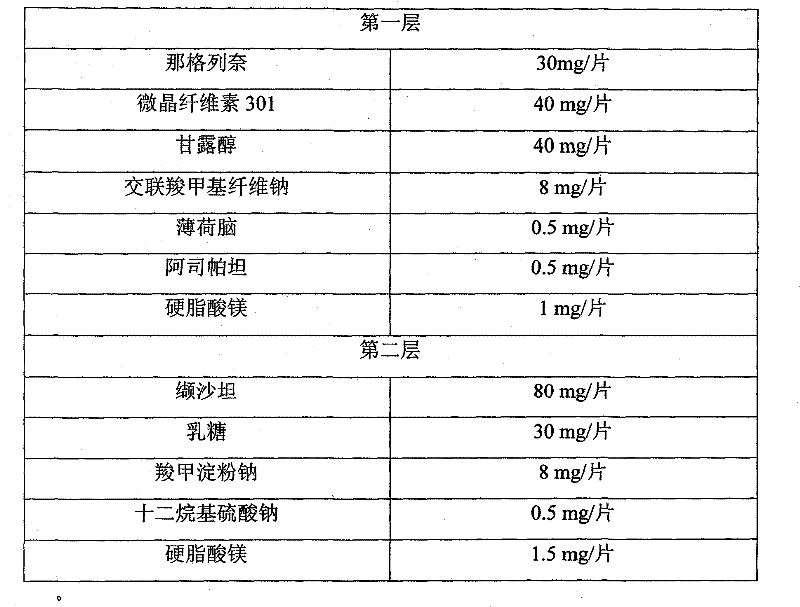
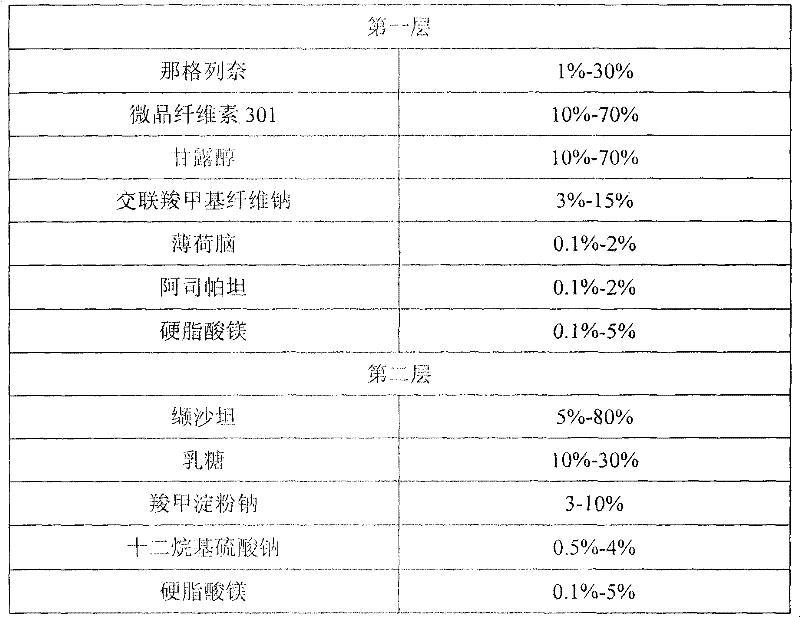
[0051] prescription composition
[0052]
[0053] Preparation Process
[0054] 1) Preparation of the first layer: Weigh nateglinide, microcrystalline cellulose 301, mannitol, menthol, aspartame, croscarmellose sodium, and mix them uniformly with 95% ethanol solution to make soft material, granulate with 12-20 mesh screen, dry at 30-70°C, granulate with 12-20 mesh screen, add magnesium stearate, mix evenly, and press the first layer.
[0055] 2) To prepare the second layer of granules, weigh valsartan, lactose, sodium carboxymethyl starch, and sodium lauryl sulfate, and mix them uniformly by adding in equal amounts, then use 50% ethanol solution to make a soft material, 12- Granulate with a 20-mesh screen, dry at 30-70°C, granulate with a 12-20-mesh screen, add magnesium stearate, mix evenly, and press the second layer.
[0056] 3) Tablet compression: After adjusting the weights of the two layers, press them into double-layer tablets with a multifunctional tablet press.
Embodiment 2
[0057] Embodiment 2 (prior art)
[0058] prescription composition
[0059] Naglinide
30mg / tablet
Microcrystalline Cellulose 301
40mg / tablet
40mg / tablet
8mg / tablet
0.5mg / tablet
0.5mg / tablet
80mg / tablet
30mg / tablet
8mg / tablet
0.5mg / tablet
2.5mg / tablet
[0060] Preparation Process
[0061] Weigh nateglinide, microcrystalline cellulose 301, mannitol, menthol, aspartame, croscarmellose sodium, valsartan, lactose, carboxymethyl starch sodium, lauryl sulfate Sodium, mix evenly by adding equal amount, make soft material with 75% ethanol solution, granulate with 12-20 mesh screen, dry at 30-70°C, granulate with 12-20 mesh screen, add magnesium stearate, Mix evenly, press into tablets, and get ready.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com