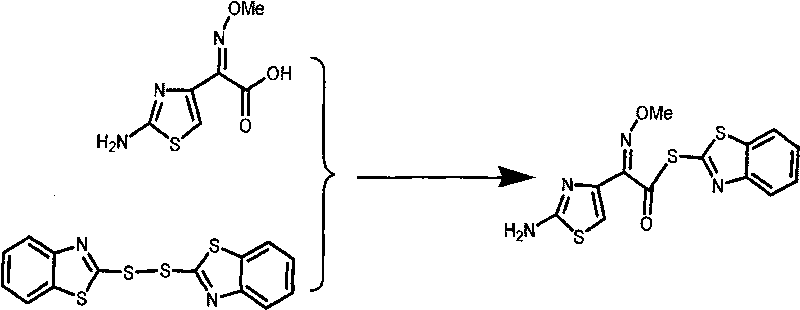
Method for synthesizing AE-active ester
A synthesis method and technology of active ester, applied in the direction of organic chemistry, etc., can solve the problems of unstable structure of AE-active ester, short storage time, storage and use restriction, etc., to achieve great implementation value and economic benefits, easier storage, lower The effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The molar ratio of the feedstocks is hydrothiazide: dithiodibenzothiazole: triethylamine: triethyl phosphite=1:1.2:1:2.5. The volume ratio of mixed solvent: dichloromethane and acetonitrile is 1.5:1. Axamic acid: glyceryl tristearate: mixed solvent = 20g: 0.6g: 150ml.
[0020] Add 20g of aminothiaxamic acid to a 500mL three-necked flask equipped with mechanical stirring, constant pressure dropping funnel and thermometer, add dithiodibenzothiazole and mixed solvent in proportion, stir well, add glyceryl tristearate, and stir at room temperature Add triethylamine and triethyl phosphite, control the temperature at 25°C to react for 3h, cool to 5°C in an ice bath, filter, wash the filter cake with methanol at 5°C, and dry under vacuum to obtain 26.33g of AE-active ester. The yield was 89%, the content was 99.1% (HPLC), and the purity was 99.57%.
Embodiment 2
[0022] The molar ratio of the feedstock is hydrothiasic acid: dithiodibenzothiazole: triethylamine: triethyl phosphite=1:1.2:1:2.5. The volume ratio of mixed solvent: dichloromethane and acetonitrile is 1.2:1. Axamic acid: glyceryl tristearate: mixed solvent = 20g: 1.6g: 150ml.
[0023] Add 20g of aminothiaxamic acid to a 500mL three-necked flask equipped with mechanical stirring, constant pressure dropping funnel and thermometer, add dithiodibenzothiazole and mixed solvent in proportion, stir well, add glyceryl tristearate, and stir at room temperature Add triethylamine and triethyl phosphite, control the temperature at 25°C to react for 3h, cool to 5°C in an ice bath, filter, wash the filter cake with methanol at 5°C, and dry in vacuum to obtain 27.51g of AE-active ester. The yield was 93%, the purity was 99.3% (HPLC), and the content was 99.8%.
Embodiment 3
[0025] The molar ratio of the feedstock is hydrothiasic acid: dithiodibenzothiazole: triethylamine: triethyl phosphite=1:1.2:1:2.5. The volume ratio of mixed solvent: dichloromethane and acetonitrile is 1.6:1. Axamic acid: glyceryl tristearate: mixed solvent = 20g: 1.2g: 200ml.
[0026] Add 20g of aminothiaxamic acid to a 500mL three-necked flask equipped with mechanical stirring, constant pressure dropping funnel and thermometer, add glyceryl tristearate and mixed solvent in proportion, stir evenly, add dithiodibenzothiazole, stir at room temperature Add triethylamine phosphite, then add triethyl, control the temperature at 25°C to react for 3h, cool to 5°C in an ice bath, filter, wash the filter cake with methanol at 5°C, and dry under vacuum to obtain 26.92g of AE-active ester. The yield was 91%, the content was 99.0% (HPLC), and the purity was 99.62%.
[0027] Compared with the traditional synthesis method, the invention has the advantages of cheap and readily available raw m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com