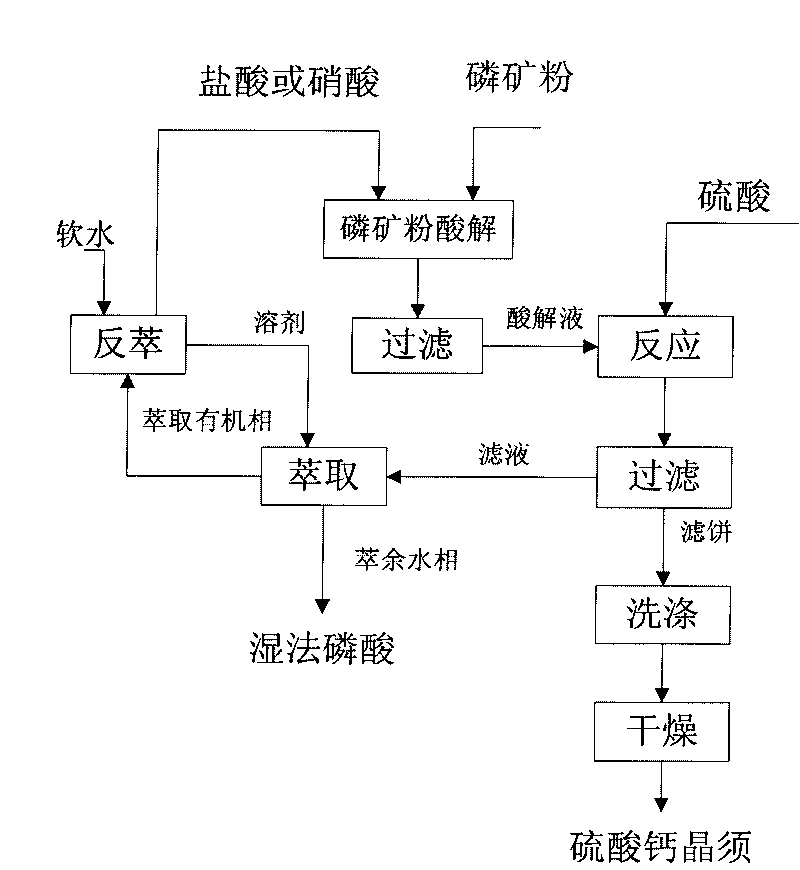
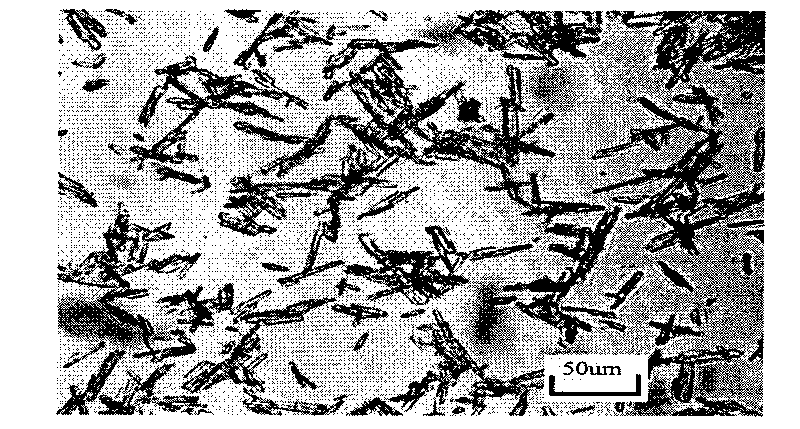
Co-production method of calcium sulfate whisker and phosphoric acid
A technology of calcium sulfate whiskers and phosphoric acid, applied in phosphoric acid, single crystal growth, crystal growth and other directions, can solve the problems of poor control of additive usage, undisclosed shape and size of calcium sulfate whiskers, and increased investment in equipment, etc. Significant economic and social benefits, the realization of no pollutant emissions, and the effect of improving resource utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The processing steps of the present embodiment are as follows:
[0043] (1) Acid hydrolysis of phosphate rock powder
[0044] The phosphate rock powder is acidolyzed with hydrochloric acid (prepared by analytical pure hydrochloric acid) with a mass concentration of 15%. The content of CaO in the phosphate rock powder is calculated as 46%. 130% of the theoretical amount; at first the hydrochloric acid is added to the reaction vessel, then rock phosphate powder is added to the hydrochloric acid under stirring, and the acid hydrolysis reaction is carried out at normal pressure and room temperature (26° C.) under stirring, and the time is 2 hours , after the reaction, the reaction slurry was allowed to stand until separated, and then obtained by suction filtration containing Ca 2+ The acid hydrolysis solution, take the acid hydrolysis solution for detection, and measure the Ca in the acid hydrolysis solution 2+ The content of is 5.70% (mass percentage).
[0045] (2) Prep...
Embodiment 2
[0050] The processing steps of the present embodiment are as follows:
[0051] (1) Acid hydrolysis of phosphate rock powder
[0052] The phosphate rock powder is acidolyzed with hydrochloric acid (prepared by analytical pure hydrochloric acid) with a mass concentration of 21%. The content of CaO in the phosphate rock powder is calculated as 46%. 110% of the theoretical amount; at first the hydrochloric acid is added to the reaction vessel, then rock phosphate powder is added to the hydrochloric acid under stirring, and the acidolysis reaction is carried out at normal pressure and room temperature (28° C.) under stirring, and the time is 1 hour , after the reaction, the reaction slurry was allowed to stand until separated, and then obtained by suction filtration containing Ca 2+ The acid hydrolysis solution, take the acid hydrolysis solution for detection, and measure the Ca in the acid hydrolysis solution 2+ The content of is 7.83% (mass percentage).
[0053] (2) Preparatio...
Embodiment 3
[0058] The processing steps of the present embodiment are as follows:
[0059] (1) Acid hydrolysis of phosphate rock powder
[0060] The phosphate rock powder is acidolyzed with hydrochloric acid (prepared by analytical pure hydrochloric acid) with a mass concentration of 26%. The content of CaO in the phosphate rock powder is calculated as 46%. 100% of the theoretical amount; first the hydrochloric acid is added to the reaction vessel, then rock phosphate powder is added to the hydrochloric acid under stirring, and the acid hydrolysis reaction is carried out at normal pressure and room temperature (26° C.) under stirring, and the time is 0.5 hours , after the reaction, the reaction slurry was allowed to stand until separated, and then obtained by suction filtration containing Ca 2+ The acid hydrolysis solution, take the acid hydrolysis solution for detection, and measure the Ca in the acid hydrolysis solution 2+ The content is 8.98% (mass percentage).
[0061] (2) Preparat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com