Method for preparing light sensitive type water soluble chitosan derivative
A technology for water-soluble chitosan and derivatives, which is applied in the field of preparation of photosensitive water-soluble chitosan derivatives, can solve the problems of troublesome post-processing, difficult to control, not conforming to the concept of environmental protection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
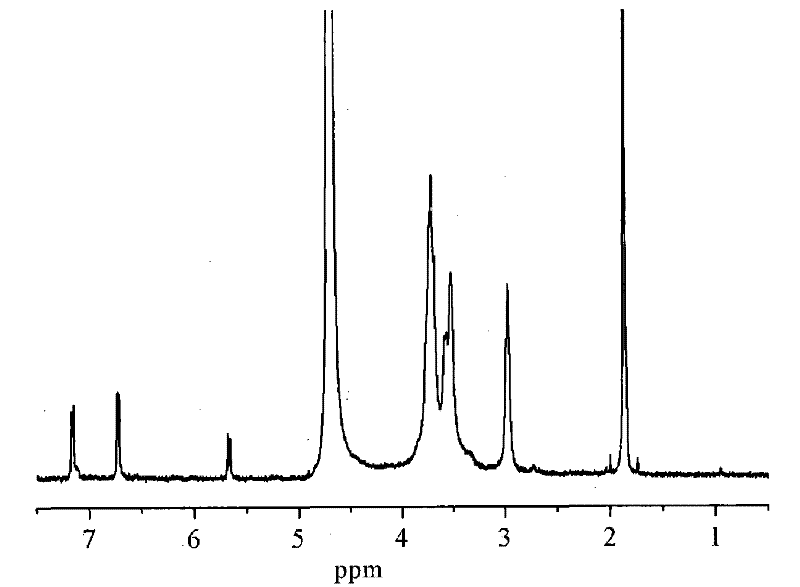
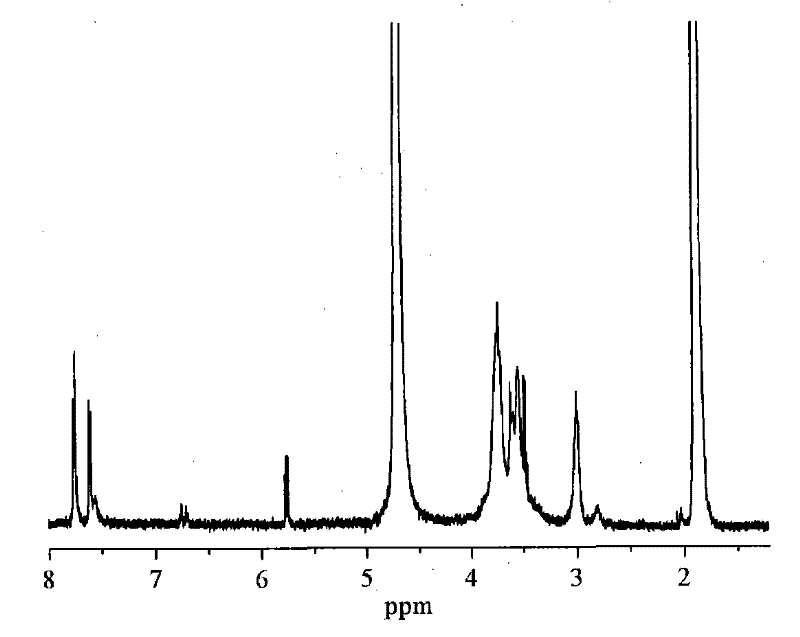
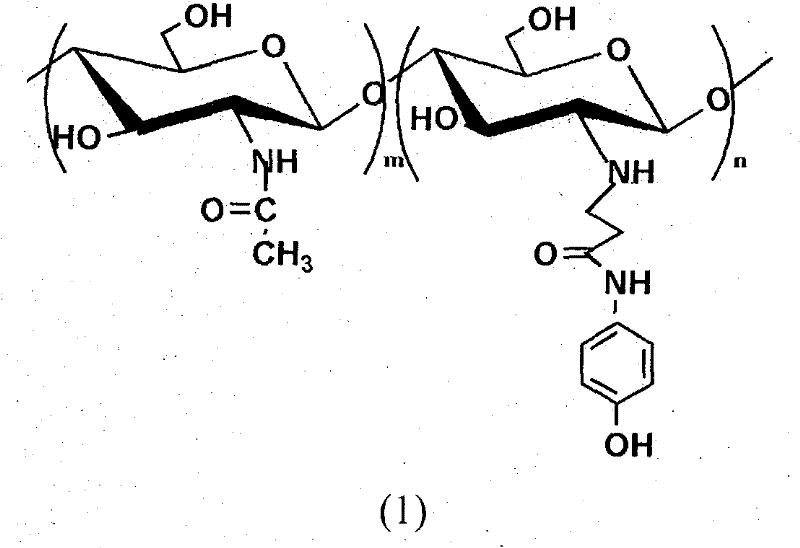
[0031] Chitosan (degree of deacetylation DP=90%, weight average molecular weight Mw=500,000) was added in the dilute solution of 2wt% acetic acid, stirred at room temperature for 0.5 hour, and it was prepared as 2% chitosan aqueous solution by weight percentage; Add the N-p-hydroxyphenylacrylamide monomer into ethanol, stir at room temperature for 0.5 hours, and prepare the ethanol solution of N-p-hydroxyphenylacrylamide, wherein the molar number of N-p-hydroxyphenylacrylamide Be 1 times of amino molar number on the chitosan; The chitosan aqueous solution that is 2% by weight and the ethanol solution of photosensitive acrylamide type monomer are mixed to obtain mixed solution; P-hydroxyanisole (its add-on is 1% of the number of moles of photosensitive acrylamide monomer) was added to the above mixed solution, and the stirring was continued at room temperature for 0.5 hours; to 50 mL, purified and vacuum-dried to obtain a photosensitive water-soluble chitosan derivative with a ...
Embodiment 2
[0036] Chitosan (degree of deacetylation DP=70%, weight average molecular weight Mw=200,000) was added in the dilute solution of 2wt% acetic acid, stirred at room temperature for 0.5 hour, and it was prepared as a 2% chitosan aqueous solution by weight percentage; Add the N-p-hydroxyphenylacrylamide monomer into ethanol, stir at room temperature for 0.5 hours, and prepare the ethanol solution of N-p-hydroxyphenylacrylamide, wherein the molar number of N-p-hydroxyphenylacrylamide Be 3 times of amino molar number on the chitosan; Be the chitosan aqueous solution of 2% by weight and the ethanol solution of photosensitive acrylamide type monomer and mix to obtain mixed solution; P-hydroxyanisole (its add-on is 3% of the moles of photosensitive acrylamide monomers) was added to the above mixed solution, and the stirring was continued for 0.5 hours at room temperature; to 50 mL, purified and vacuum-dried to obtain a photosensitive water-soluble chitosan derivative with a degree of s...
Embodiment 3
[0041]Chitosan (degree of deacetylation DP=50%, weight average molecular weight Mw=1,000,000) was added in 2wt% dilute acetic acid solution, stirred at room temperature for 0.5 hour, and prepared into a 2% aqueous solution of chitosan by weight; Add the N-p-hydroxyphenylacrylamide monomer into ethanol, stir at room temperature for 0.5 hours, and prepare the ethanol solution of N-p-hydroxyphenylacrylamide, wherein the molar number of N-p-hydroxyphenylacrylamide Be 4 times of the molar number of amino groups on the chitosan; Mix the ethanol solution that is the chitosan aqueous solution of 2% and the photosensitive acrylamide type monomer by weight percentage to obtain mixed solution; P-hydroxyanisole (its add-on is 4% of the moles of photosensitive acrylamide monomers) was added to the above mixed solution, and the stirring was continued for 0.5 hours at room temperature; to 50 mL, purified and vacuum-dried to obtain a photosensitive water-soluble chitosan derivative with a sub...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of deacetylation | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com