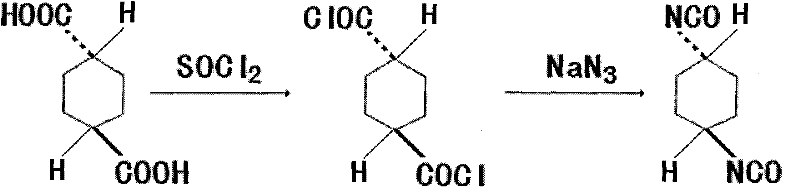
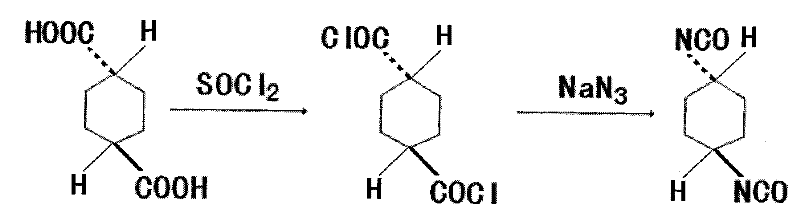
Synthetic method of trans-1,4-cyclohexane diisocyanate
A technology of cyclohexane diisocyanate and cyclohexane dicarboxylic acid, applied in the preparation of carboxylic acid nitrogen-containing derivatives, organic chemistry, etc., can solve the problems of short reaction cycle, achieve short reaction cycle, easy to obtain raw materials, and process route simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 172.18g trans-1,4-cyclohexanedicarboxylic acid (1mol), 440ml (6mol) thionyl chloride, add in the three-necked flask of reflux condenser, stirring, thermometer and tail gas absorption device, heat and reflux for 4 hours, The excess thionyl chloride was recovered under reduced pressure to obtain 199.5 g of crude trans-1,4-cyclohexanedicarbonyl chloride, with a yield of 94.99%.
Embodiment 2
[0020] 172.18g trans-1,4-cyclohexanedicarboxylic acid (1mol), 440ml (6mol) thionyl chloride, add in the three-necked flask of reflux condenser, stirring, thermometer and tail gas absorption device, heat and reflux for 3.5 hours, The excess thionyl chloride was recovered under reduced pressure to obtain 196.9 g of crude trans-1,4-cyclohexanedicarbonyl chloride, with a yield of 94.21%.
Embodiment 3
[0022] 172.18g trans-1,4-cyclohexanedicarboxylic acid (1mol), 440ml (6mol) thionyl chloride, add in the three-necked flask of reflux condenser, stirring, thermometer and tail gas absorption device, heat and reflux for 3 hours, The excess thionyl chloride was recovered under reduced pressure to obtain 186.7 g of crude trans-1,4-cyclohexanedicarbonyl chloride, with a yield of 89.33%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| softening point | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com