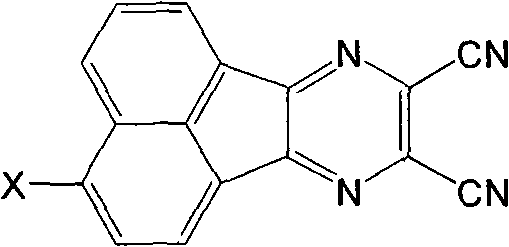
O-dicyano-acenaphtho pyrazine compound and anti-tumor application thereof
A compound, adjacent two technology, applied in antitumor drugs, organic chemistry, drug combination and other directions, can solve the problems of low solubility, difficult testing, low yield and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the synthesis of 5-bromoacenaphthenequinone (2)
[0037] In a 500mL two-necked flask, add 20g (109.8mmol) of acenaphthylquinone and 25.0mL of liquid bromine (466.8mmol), start stirring, control the temperature in an oil bath at 60-70°C, and reflux the reaction solution. After 2 hours, stop the reaction and add Sodium bisulfate aqueous solution until the reaction solution is colorless. After diluting with water, filter under reduced pressure, and wash with water for several times for beating until pH=7.0. The filter cake was recrystallized four times in glacial acetic acid to obtain 5-bromoacenaphthoquinone as brown-yellow needle-like crystals with a yield of 90%.
[0038] 1 H NMR (400MHz, DMSO) δ (ppm) 8.39(d, 1H), 8.21(d, 1H), 8.15(d, 1H), 8.04(t, 1H), 7.96(d, 1H).
Embodiment 2
[0039] Example 2: Synthesis of 3-bromo-acenaphthopyrazine-8,9 dinitrile (3)
[0040] Add 200mg (0.77mmol) of 5-bromoacenaphthylquinone and 110mg (1.07mmol) of diaminomaleonitrile into a 15ml two-necked flask, then add 10ml of glacial acetic acid, stir and heat under reflux for 2.0h, pour into water after cooling, filter, and dry use CH 2 Cl 2 : Petroleum ether=3: 1 (volume ratio) solution is that eluent carries out separation and purification on silica gel column, obtains bright yellow 3-bromo-acenaphthopyrazine-8, 9 dinitriles 192mg (0.58mmol), yield 67.7%.
[0041] 1 H NMR (400MHz, CDCl 3)δ (ppm) 8.03-8.06(t, 1H), 8.18-8.19(d, 1H), 8.34-8.35(d, 1H), 8.48-8.50(d, 1H), 8.56-8.57(d, 1H).
Embodiment 3
[0042] Example 3: Synthesis of 3-(2-dimethyl)ethylamino-acenaphthopyrazine-8,9 dinitrile (4a)
[0043] Take 100 mg (0.3 mmol) of 3-bromo-acenaphthopyrazine-8,9 dicarbonitrile in a 10 ml round bottom flask, add 5 ml of ethylene glycol monoether, and add 234 μl (2.0 mmol) of ethylene glycol monoether under stirring, N 2 Heated under protection and refluxed for 45min, the solution gradually changed from light yellow to dark red. After cooling, it was poured into water and filtered to obtain a red solid. After drying, dichloromethane:methanol:triethylamine=100:2.5:1 (volume ratio) as the eluent to carry out separation and purification on a silica gel column to obtain red powdery solid 3-(2-dimethyl) ethylamino-acenaphthopyrazine-8, 9 dinitrile 50mg (0.15mmol), yield 50.0%.
[0044] 1 H NMR (400MHz DMSO) δ (ppm) 8.78-8.76 (d, 1H), 8.54-8.52 (d, 1H), 8.36 (t, 1H), 8.31-8.29 (d, 1H), 7.89-8.91 (t, 1H), 6.93-6.95(d, 1H), 3.64-3.66(m, 2H), 2.84(t, 2H), 2.42(s, 6H); HRMS(EI) m / z(M+H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com