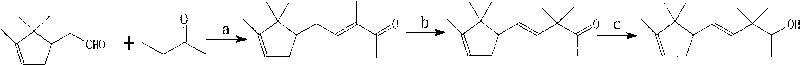Method for synthesizing polysantalol
A technology of polysantalol and synthesis method, applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve the problems of low reaction yield and complicated operation, achieve high activity and improve reaction yield , Easy to operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0007] The present invention is further illustrated below by way of examples.
[0008] Synthesis of Polysantalol: In the first step, 3-methyl-5-(2,2,3-trimethyl-3-cyclopenten-1-yl) is obtained by condensation reaction of bornene aldehyde and butanone -3-penten-2-one; second step, 3-methyl-5-(2,2,3-trimethyl-3-cyclopenten-1-yl)-3-penten-2- Addition reaction of ketone with methyl iodide to produce 3,3-dimethyl-5-(2,2,3-trimethyl-3-cyclopenten-1-yl)-4-pentene-2- Ketone; the third step, 3,3-dimethyl-5-(2,2,3-trimethyl-3-cyclopenten-1-yl)-4-penten-2-ketone reduction makes poly Sandalol. Its reaction formula is as follows:
[0009]
[0010] a: potassium hydroxide, methanol-water, room temperature, sulfuric acid; b: sodium hydroxide, octadecyltrimethylammonium bromide, toluene, methyl iodide; c: potassium borohydride, ethanol.
[0011] Concrete preparation steps of the present invention:
[0012] The first step: the preparation of 3-methyl-5-(2,2,3-trimethyl-3-cyclopenten-1-y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com