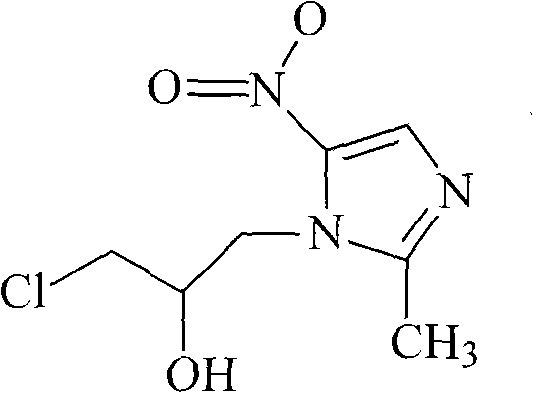
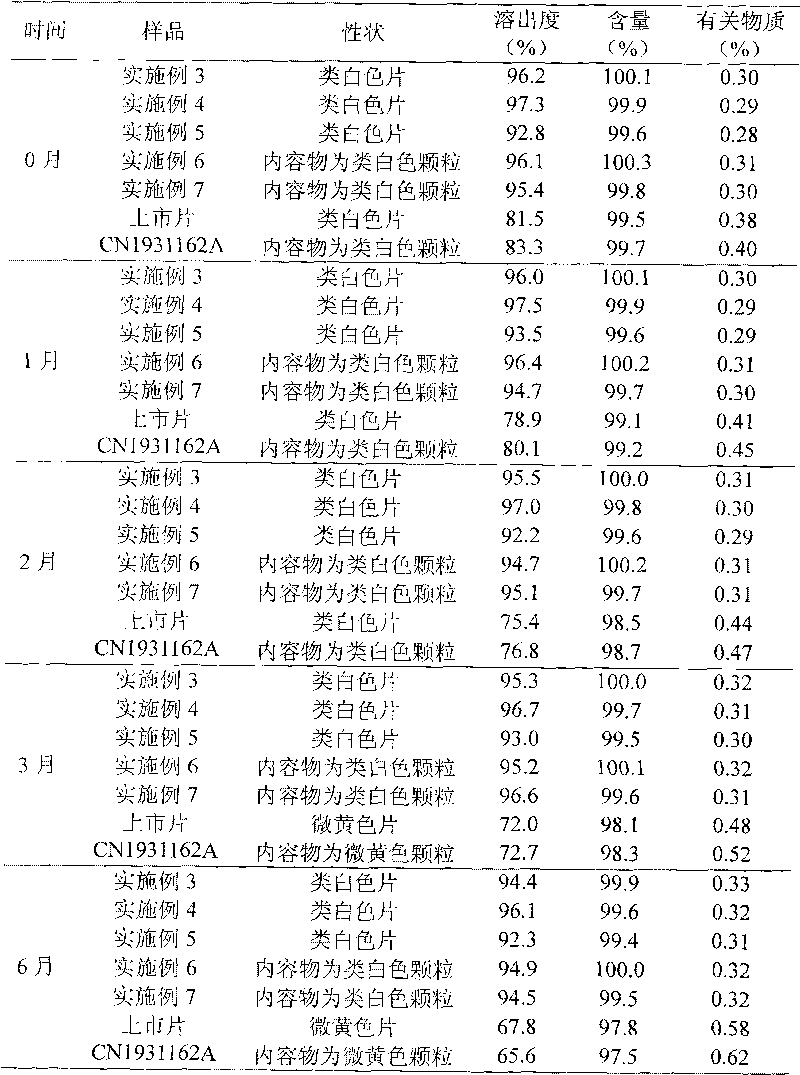
Ornidazole ester microsphere solid preparation
A technology of solid preparation and ornidazole ester, applied in the field of medicine, can solve the problems of loss of drug efficacy, poor solubility of ornidazole, and reduced bioavailability, and achieve the effects of reducing drug side effects, improving solubility and high dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1 Preparation of Ornidazole Lipid Microspheres
[0070] Prescription: Ornidazole 100g
[0071] Hydrogenated egg yolk lecithin 330g
[0072] Cholesterol 140g
[0073] Poloxamer 188 180g
[0074] Sodium Glycocholate 220g
[0075] Preparation Process
[0076] (1) 100g ornidazole, 330g hydrogenated egg yolk lecithin, 140g cholesterol, 180g poloxamer 188, 220g sodium glycocholate are dissolved in 4000ml volume ratio of acetone and isopropanol mixed solvent of 1:3, Mix evenly, and remove the mixed solvent under reduced pressure on a rotary thin film evaporator to obtain a phospholipid film;
[0077] (2) Add 2500ml of acetic acid-sodium acetate buffer solution with a pH value of 4.5, shake, stir for 20min, and rotate at a speed of 600r / min to completely hydrate the phospholipid film, then homogeneously emulsify with a tissue masher for 10min at a speed of 15000r / min, and then Filter with a 0.45 μm microporous membrane to prepare a suspe...
Embodiment 2
[0086] Example 2 Preparation of Ornidazole Lipid Microspheres
[0087] Prescription: Ornidazole 100g
[0088] Hydrogenated egg yolk lecithin 850g
[0089] Cholesterol 530g
[0090] Poloxamer 188 400g
[0091] Sodium Glycocholate 350g
[0092] Preparation Process
[0093] (1) 100g ornidazole, 850g hydrogenated egg yolk lecithin, 530g cholesterol, 400g poloxamer 188, 350g sodium glycocholate are dissolved in 15000ml volume ratio of acetone and isopropanol mixed solvent of 1:3, Mix evenly, and remove the mixed solvent under reduced pressure on a rotary thin film evaporator to obtain a phospholipid film;
[0094] (2) Add 11,000ml of citric acid-sodium citrate buffer solution with a pH value of 6.5, shake, stir for 30min, and rotate at a speed of 200r / min to completely hydrate the phospholipid film, then use a tissue masher to homogeneously emulsify for 20min, and rotate at a speed of 200r / min. 13000 / min, and then filtered through a 0.45 μm micropo...
Embodiment 3
[0103] Example 3 Preparation of Ornidazole Tablets
[0104] Prescription (100 tablets)
[0105] Ornidazole lipid microspheres (calculated as ornidazole) 25g
[0106] Starch 5g
[0107] Microcrystalline Cellulose 4.5g
[0108] Sodium starch glycolate 1.5g
[0109] Povidone K30 2g
[0110] Magnesium stearate 0.5g
[0111] Preparation Process
[0112] (1) Pulverize the lipid microspheres containing 25g ornidazole, cross 80 mesh sieves, and set aside;
[0113] (2) 5g starch, 4.5g microcrystalline cellulose, and 1.5g carboxymethyl starch sodium are passed through an 80-mesh sieve, mixed, and set aside;
[0114] (3) Prepare 5% Povidone K 30 100ml of 80% ethanol solution, set aside;
[0115] (4) Mix the above raw and auxiliary materials evenly, add 5% povidone K 30 40ml of 80% ethanol solution to make soft material, granulate through 20 mesh sieve, and dry at 60°C;
[0116] (5) Add 0.5 g of magnesium stearate to the dried granules and mix evenly, sieve the granules wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com