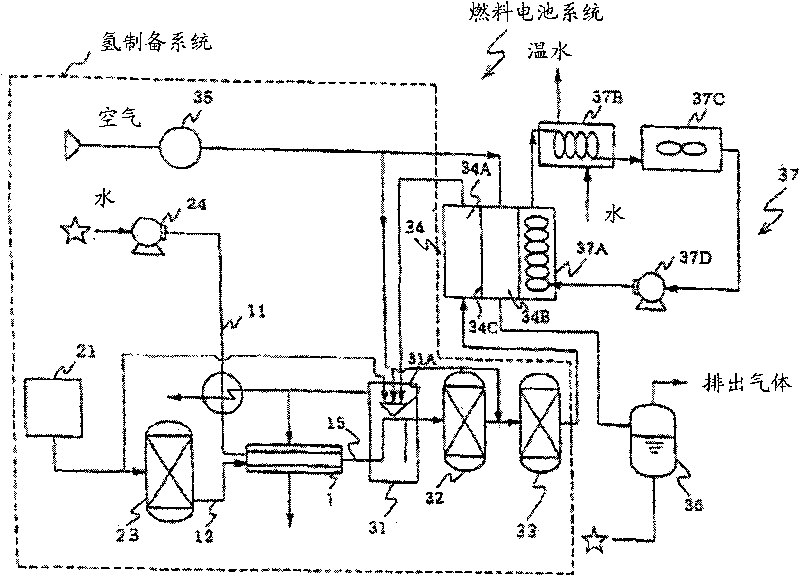
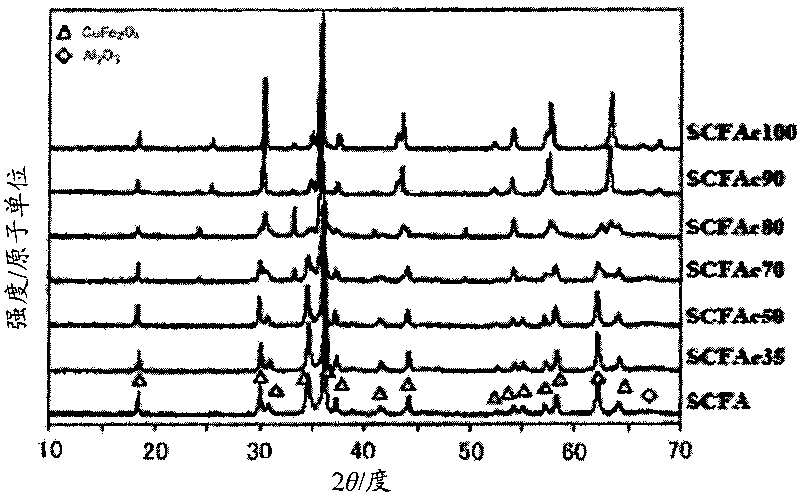
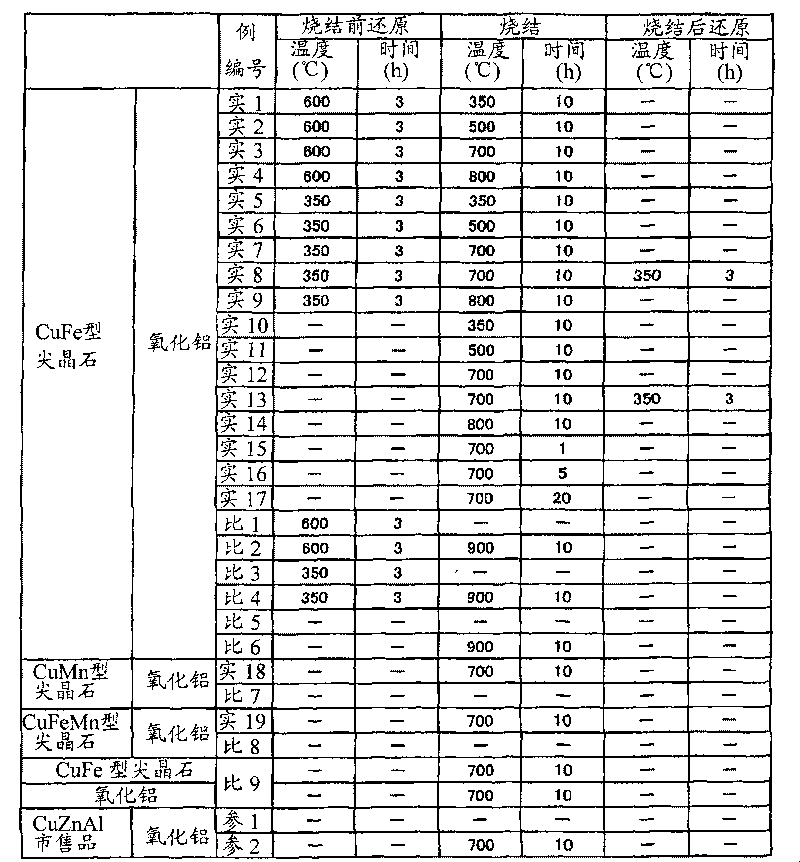
Catalyst for reforming oxygen-containing hydrocarbon, and hydrogen or synthetic gas production method and fuel cell system using the catalyst
A synthesis gas and catalyst technology, applied in catalyst activation/preparation, fuel cell, hydrogen/synthesis gas production, etc., can solve problems such as catalyst deactivation, and achieve the effects of improved durability and excellent reforming activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0134] Preparation Example 1CuFe 2 O 4 Spinel oxide
[0135] 24.184g copper nitrate (manufactured by Wako Pure Chemical Industries, Ltd., 99.9% Cu(NO 3 ) 2 ·3H 2 O) and 80.881g iron nitrate (manufactured by Wako Pure Chemical Industries, Ltd., 99.9% Fe(NO 3 ) 3 ·9H 2 O) Put it into a beaker, add distilled water to dissolve it, and make the volume 300ml. It was heated to 60°C and stirred for 2 hours.
[0136] Then, 92.926 g of citric acid monohydrate (manufactured by Wako Pure Chemical Industries, Ltd., 99.5% C 6 H 8 O 7 ·3H 2 O), further stirring at 60°C for 1 hour, and then raising the temperature to 90°C to evaporate water.
[0137] The nitrate and citric acid of the gel produced in this way are decomposed in air at 140-200°C to obtain fine oxide powder, and then sintered in air at 900°C for 10 hours to obtain CuFe 2 O 4 Spinel type oxide.
preparation example 2C
[0138] Preparation Example 2CuMn 2 O 4 Spinel oxide
[0139] 24.184g copper nitrate (manufactured by Wako Pure Chemical Industries, Ltd., 99.9% Cu(NO 3 ) 2 ·3H 2 O) and 58.588g of manganese nitrate (manufactured by Aldrich, 98% Mn(NO 3 ) 2 ·6H 2 O) Put it into a beaker, add distilled water to dissolve it, and make the volume 300ml. It was heated to 60°C and stirred for 2 hours.
[0140] Then, 92.926 g of citric acid monohydrate (manufactured by Wako Pure Chemical Industries, Ltd., 99.5% C 6 H 8 O 7 ·3H 2 O), further stirring at 60°C for 1 hour, and then raising the temperature to 90°C to evaporate water.
[0141] The nitrate and citric acid of the gel produced in this way are decomposed in air at 140-200°C to obtain fine oxide powder, and then sintered in air at 900°C for 10 hours to obtain CuMn 2 O 4 Spinel type oxide.
preparation example 3
[0142] Preparation Example 3CuFe 1.5 Mn 0.5 O 4 Spinel oxide
[0143] 24.184g copper nitrate (manufactured by Wako Pure Chemical Industries, Ltd., 99.9% Cu(NO 3 ) 2 ·3H 2 O), 60.661g iron nitrate (manufactured by Wako Pure Chemical Industries, Ltd., 99.9% Fe(NO 3 ) 3 ·9H 2 O) and 14.647g manganese nitrate (manufactured by Aldrich, 98% Mn(NO 3 ) 2 ·6H 2 O) Put it into a beaker, add distilled water to dissolve it, and make the volume 300ml. It was heated to 60°C and stirred for 2 hours.
[0144] Then, 92.926 g of citric acid monohydrate (manufactured by Wako Pure Chemical Industries, Ltd., 99.5% C 6 H 8 O 7 ·3H 2 O), further stirring at 60°C for 1 hour, and then raising the temperature to 90°C to evaporate water.
[0145] The nitrate and citric acid of the gel produced in this way are decomposed in air at 140-200°C to obtain fine oxide powder, and then sintered in air at 900°C for 10 hours to obtain CuFe 1.5 Mn 0.5 O 4 Spinel type oxide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com