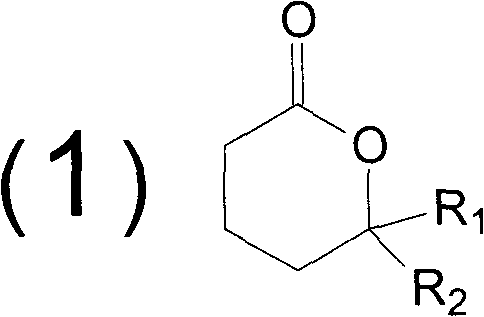
Method for synthesizing butyrolactone and butyrolactone obtained by same
A kind of lactone and its ester technology are applied in the field of synthesizing butyl lactone and the butyl lactone obtained therefrom, and can solve the problems of many three wastes, complicated process, long synthesis steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Synthesis of butyl-decalactone (δ-n-pentyl-δ-valerolactone)
[0040] In a reactor equipped with a stirrer, a condenser, a thermometer and a dropping device, 306 g (3 moles) of 1-hexanol was added, and it was heated to 150°C. The mixed solution of 100 grams (1 mole) of methyl 3-butenoate and 97 grams (0.5 moles) of tert-butyl peroxybenzoate was added dropwise in one hour, and the reaction was continued at this temperature for 3 hours after the addition was completed, and then cooled to At room temperature, excess 1-hexanol was recovered, and then rectified under reduced pressure to obtain 132.6 g of butyl-decalactone, and the calculated yield was 78%.
Embodiment 2~ Embodiment 20
[0042] Using the types and amounts of alcohol, 3-butenoic acid or its ester, and initiator shown in Table 1, and appropriately changing the reaction temperature, other conditions were the same as those in Example 1 to prepare the corresponding δ-substituted-δ-pentene ester. The yields are also shown in Table 1 together.
[0043] Table 1
[0044]
[0045]
[0046]
[0047] As can be seen from the above Table 1, according to the synthesis method of the present invention, a high yield of δ-substituted-δ-valerolactone can be obtained.
Embodiment 21~ Embodiment 27
[0049] As shown in Table 2, the amounts of 1-hexanol, methyl 3-butenoate, and tert-butyl peroxybenzoate and the reaction temperature were changed, and other conditions were the same as in Example 1 to prepare butyl-decalactone. The yields are also shown in Table 2 together.
[0050] Table 2
[0051]
[0052] As can be known from the above table 2, when the amount of 1-hexanol, methyl 3-butenoate, tert-butyl peroxybenzoate and the reaction temperature are not in the preferred range specified by the present invention, although the target product can also be obtained, but The yield is not very high.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com