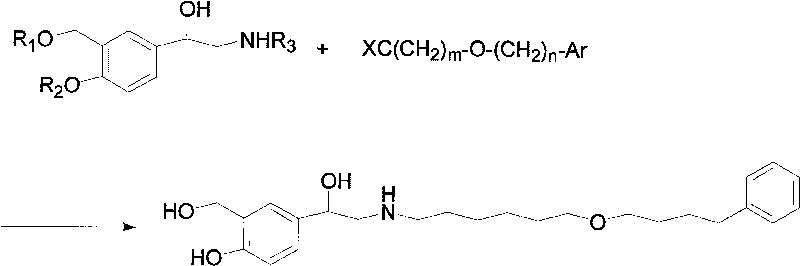
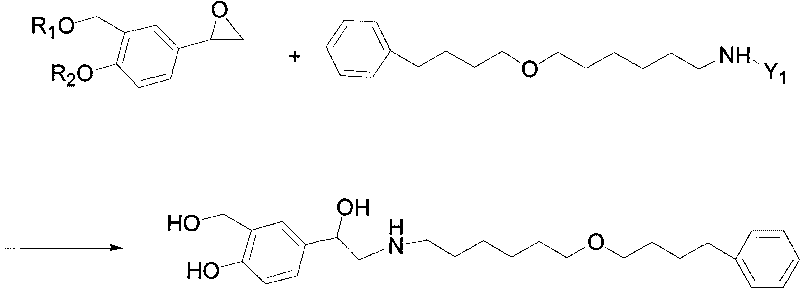
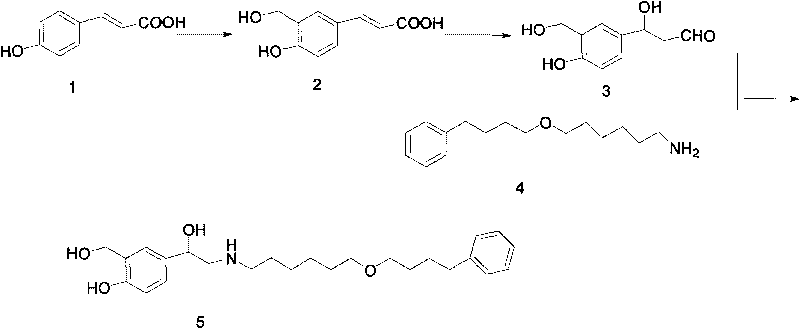
Method for preparing anti-asthmatic medicament of salmeterol
A drug and asthma technology, which is applied in the field of preparation of the anti-asthma drug salmeterol, can solve the problems of cumbersome preparation method, unsatisfactory yield, and high difficulty in industrialization, and achieve convenient experimental operation, novel synthetic route and low production cost. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0022] The specific preparation steps of the present invention's synthetic Mexaterol are as follows: (1) Synthesis of 3-hydroxymethyl-4-hydroxyl-phenylacrylic acid (2): add 200ml p-xylene in a 500ml single-necked round-bottomed flask, then add 32.8 Gram (0.2mol) of p-hydroxyphenylacrylic acid, 50ml of formaldehyde, 25 grams of boric acid (0.4mol), then heated to 150°C and refluxed for 12h; then cooled to room temperature to precipitate a large amount of yellow precipitate, filtered, the obtained precipitate was washed with an appropriate amount of water, and then dried to obtain The product 3-hydroxymethyl-4-hydroxy-phenylacrylic acid was 28.2 grams, the yield was 80%, and the melting point (mp): 185.1-185.7°C.
[0023] (2) Synthesis of 3-hydroxymethyl-4-hydroxyl-(b-hydroxyl)-phenylpropanal (3): add 19.4 grams (0.1mol) of 3- Hydroxymethyl-4-hydroxy-phenylacrylic acid, 50ml of 30% fresh hydrogen peroxide (0.4mol), 0.2g of lipase and 150ml of ethyl acetate as a reaction solvent,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com