Method for preparing glucocorticoid grafted gene drug delivery vector
A technology of glucocorticoids and drug delivery carriers, which is applied in gene therapy, pharmaceutical formulations, liposome delivery, etc., can solve the problems of low biocompatibility, achieve simple preparation process, reduce toxicity, and improve transfection efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Dissolve 10 mg of phospholipids and 0 to 4.83 mg of cholesterol in 15 mL of chloroform, and dissolve 3.2 to 67.45 mg of glucocorticoid-grafted polycations in distilled water (5 mL) that is 1 / 3 of the volume of chloroform, and prepare lipids by reverse evaporation. body. Specifically: add the water phase to the organic phase, vortex or sonicate for 10 to 30 minutes to form an emulsion, and remove chloroform under reduced pressure on a rotary evaporator to obtain a liposome suspension. The liposome solution is sonicated in a water bath for 5-20 minutes, squeezed through the membrane, and the pore size is 0.1-0.22 μm.
Embodiment 2
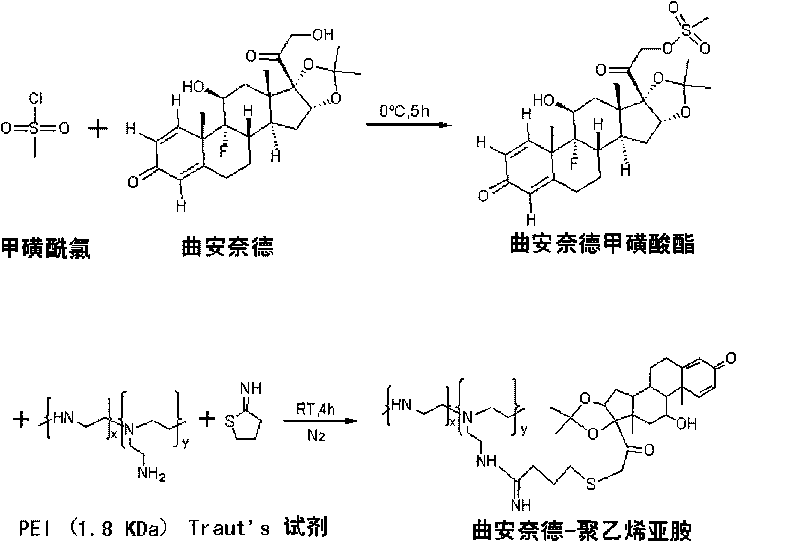
[0031] Take triamcinolone acetonide 217.25mg and dissolve it with anhydrous pyridine, and the solution is at 0°C, N 2 Under protection, under stirring, slowly add 78 μL of excess methanesulfonyl chloride dropwise, react at 0°C for 5 hours, add excess ice water (about 80 mL) at 0°C to the reaction solution to terminate the reaction, filter with suction, wash with ice water, and dry in vacuo. A white solid was obtained, which was the 21-position mesylate of triamcinolone acetonide.
[0032] Dissolve 133.9 mg of triamcinolone acetonide 21-position mesylate in excess of 4 times and Traut’s reagent 34.4 mg in 2 mL of anhydrous DMSO, and dissolve 112.5 mg of low molecular weight PEI 1800 in 2 mL of anhydrous DMSO. 2 Add dropwise to the former solution under protection and stir while adding. The reaction was carried out at room temperature for 4 hours, then 80 mL of excess iced ethyl acetate was added, and a pale yellow precipitate was obtained by filtration, which was dissolved by ...
Embodiment 3
[0033] Embodiment 3: NMR characterization of triamcinolone acetonide 21-position mesylate and triamcinolone acetonide-polyethyleneimine (TA-PEI)
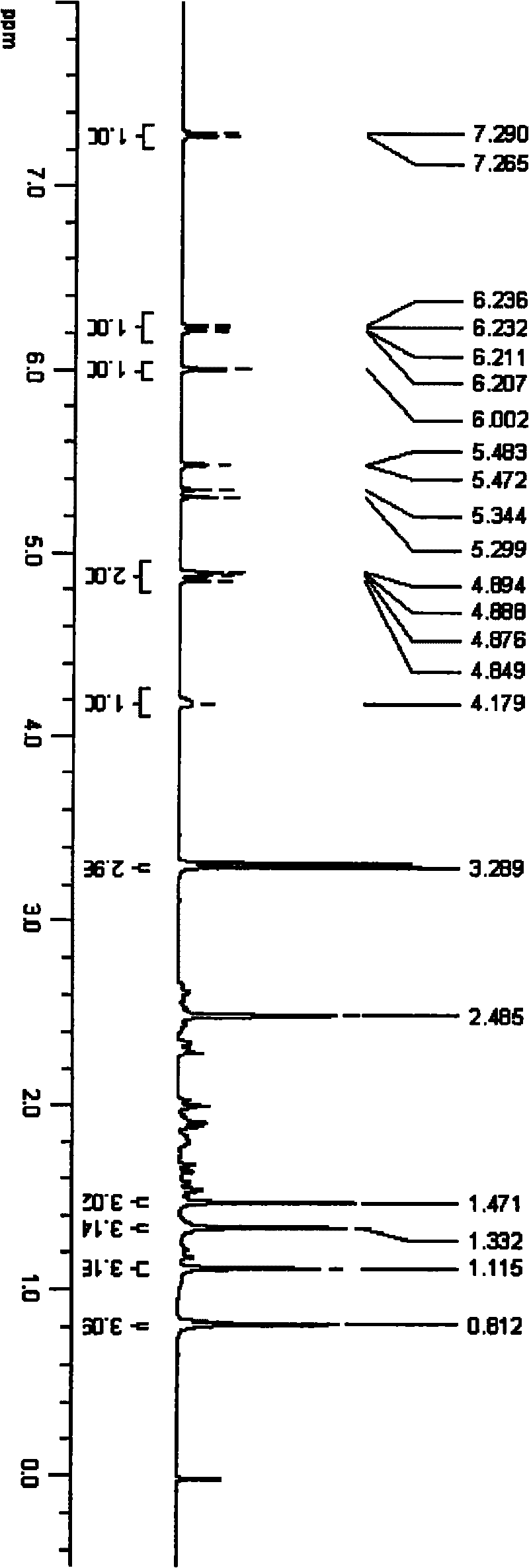
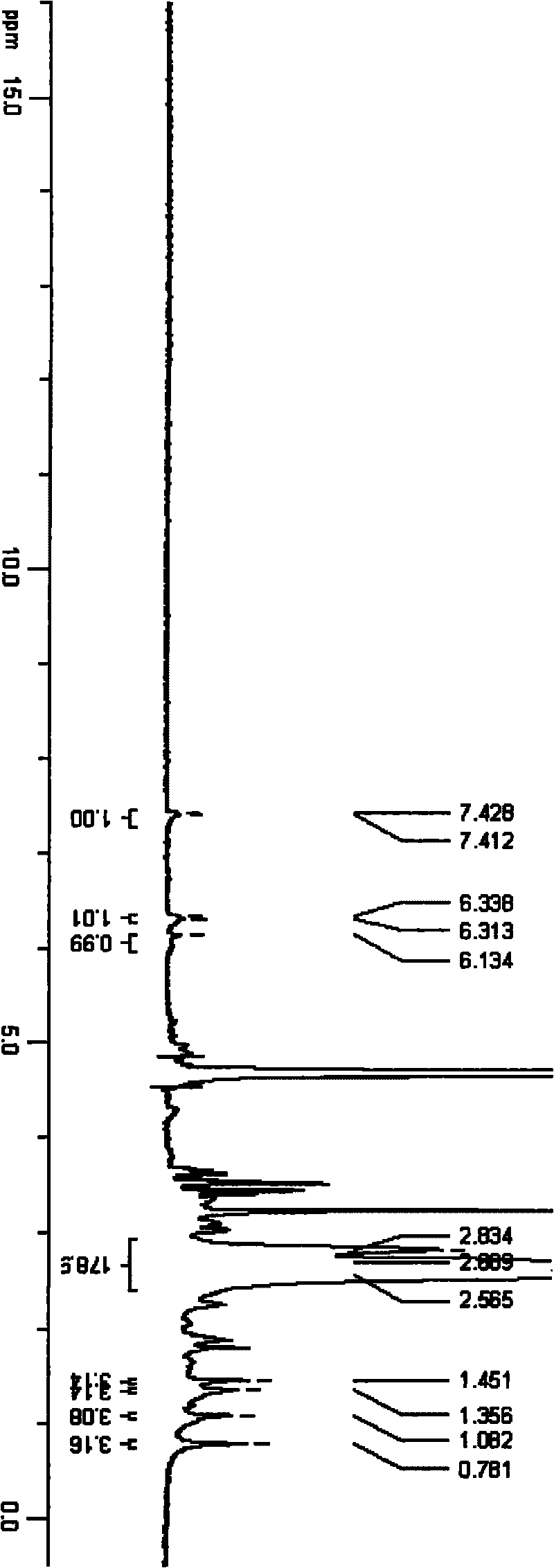
[0034] An appropriate amount of triamcinolone acetonide 21-position mesylate was dissolved in deuterated DMSO, and 500MHz 1H-NMR scanning was performed. An appropriate amount of TA-PEI was dissolved in deuterium water, and a 500MHz 1H-NMR scan was performed. Please refer to the attached figure 2 . See the attached image 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com