Application of cerebroside compound
A technology for cerebrosides and compounds is applied in the application field of preparing medicines for treating post-ischemic brain injury, which can solve the problems of difficulty in separation and purification, and achieve the effects of significant therapeutic effect, reduction of cerebral infarction area and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The method for preparing cerebroside A and cerebroside B from Gallus pilosulae, the concrete steps are:
[0033] (1) Pulverization: the dried Gallinarum gallinarum sample (1.5kg) (Yanyuan County, Sichuan Province) is pulverized to 100~10 orders;
[0034] (2) Solvent extraction: the pulverized gallinaceous fungus is packed into a 15-liter container, and methanol (7.5 liters) is shaken and extracted in a shaker for 3 days to obtain the gallinaceous fungus extract (151.3g); Subsequently, the extract was distributed with 90% aq.MeOH and n-hexane, and the resulting 90% aq.MeOH solution was concentrated and then distributed with water and n-butanol, and the n-butanol solution was concentrated under reduced pressure to obtain n-butanol layer sample (16.0 g);
[0035] (3) separation and purification: n-butanol layer sample with ODS open column (solvent system is MeOH: H 2 (0=90:10, 95:5, 100:0) separation; then, the sample containing the target compound (95% aq.MeOH fraction,...
Embodiment 2
[0037] The physicochemical characteristics and the qualitative identification of chemical structure of cerebroside A and cerebroside B that embodiment 1 obtains:
[0038] Physicochemical properties of cerebroside A: [α] 25 D+4.5 (c 0.46, MeOH); Infrared spectrum (KBr) values: 3371, 2921, 2853, 1643, 1536, 1468, and 1081cm-1; m / z 728 (M+H)+;, chemical shift value: C (150MHz, DMSO+2%D2O): 173.91, 135.08, 131.26, 131.05, 123.61, 103.56, 77.00, 76.63(76.62, CH-OD), 73.49(73.46), 71.20(71.17), 70.81(70.71), 70.25( 70.20), 68.86, 61.33 (61.21), 53.04 (53.00, CH-ND-), 39.90, 34.53, 32.23, 31.36, 29.10, 28.98, 28.74, 27.49, 27.38, 24.61, 22.14, 15.83, and 13.95.
[0039] Cerebroside B: The chemical structure of cerebroside B was determined using the same method as that of cerebroside A, and it was found that the difference between cerebroside B and A in structure (including three-dimensional structure) is that B is in the fatty chain There are two more methylene groups than A. Phy...
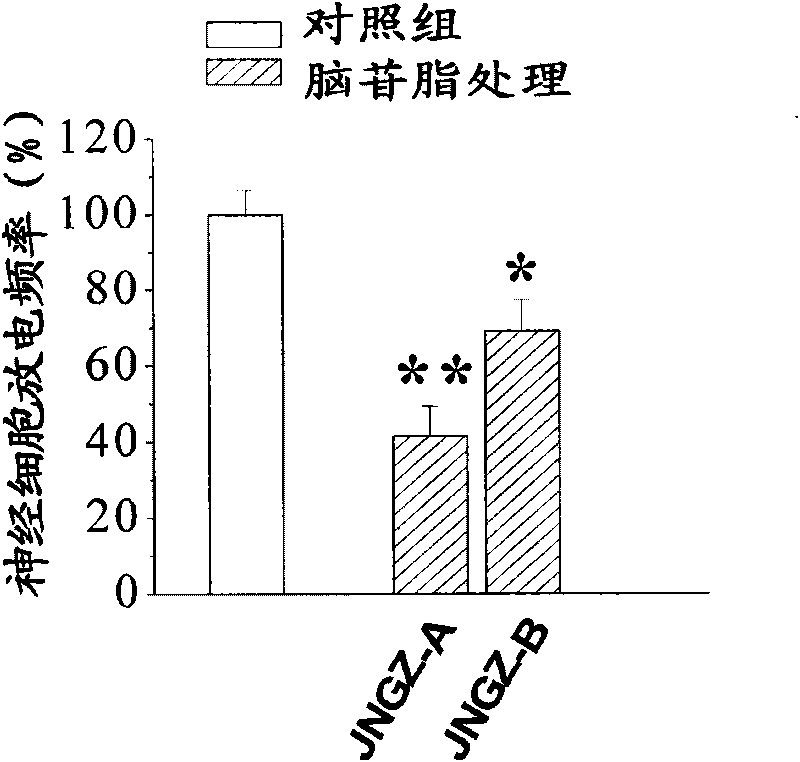
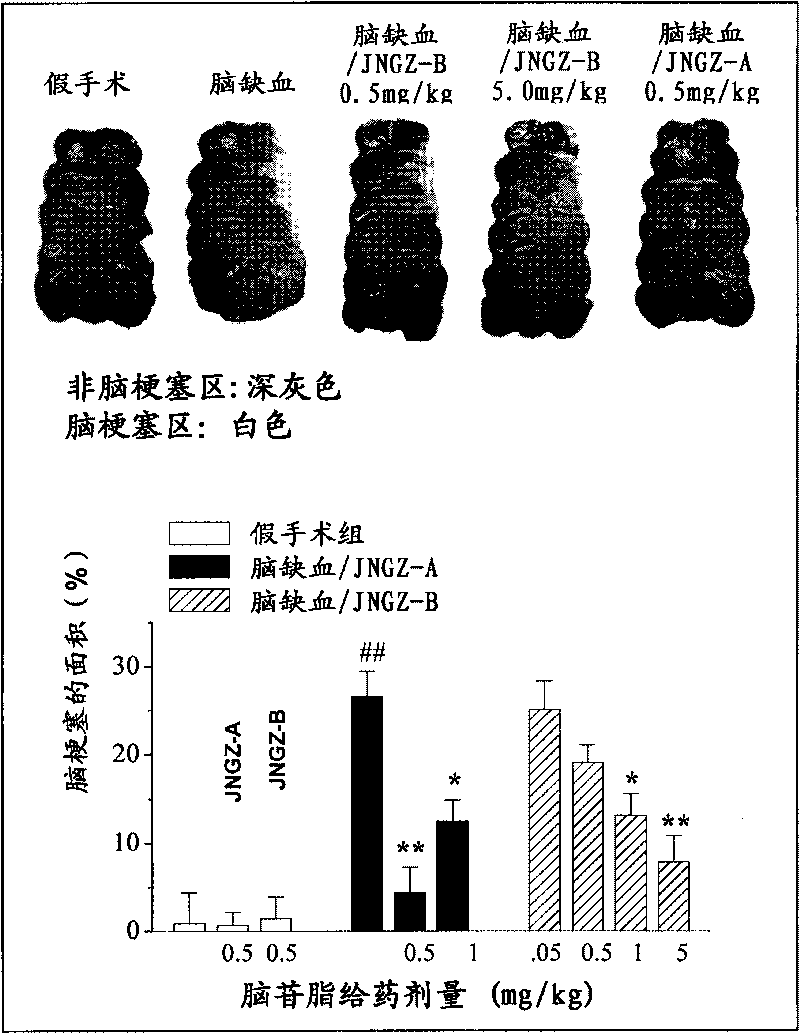
Embodiment 3
[0041] The animal pharmacology experiments of cerebroside A (JNGZ-A) and cerebroside B (JNGZ-B) described in the present invention are carried out to prove their application in the preparation of medicines for treating brain injury after ischemia.
[0042] The degree of neurological damage due to different forms of cerebral ischemia varies. The main pathological manifestations of middle cerebral artery occlusion are infarction and cerebral edema in the striatum, cerebral cortex, and hippocampus, which have a very high mortality and disability rate. Global cerebral ischemia selectively damages central nervous cells sensitive to hypoxia (such as nerve cells in the CA1 area of the hippocampus), resulting in memory loss. Therefore, the present invention will use the classic middle cerebral artery occlusion (middle cerebral artery occlusion, MCAO) operation to simulate cerebral apoplexy, establish an animal model of global cerebral ischemia with vertebral artery and common caroti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com