Method for preparing gonadorelin freeze-dried powder injection
The technology of freeze-dried powder injection and freeze-drying machine, which is applied in the field of medicine, can solve the problems of large difference in pH value, unstable product quality, and reduced product content, and achieves improved stability, shortened freeze-drying time, and stable product quality. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
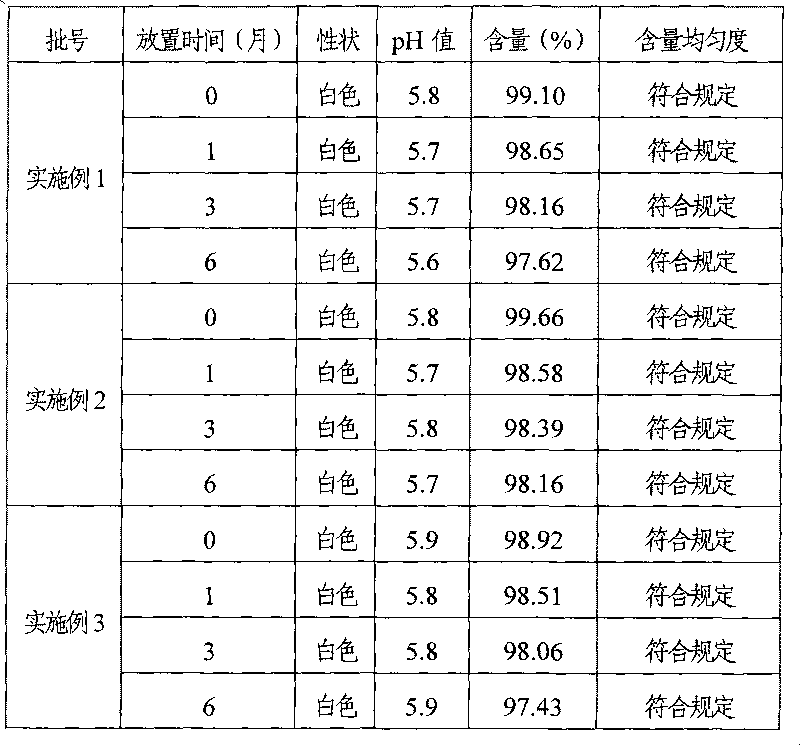
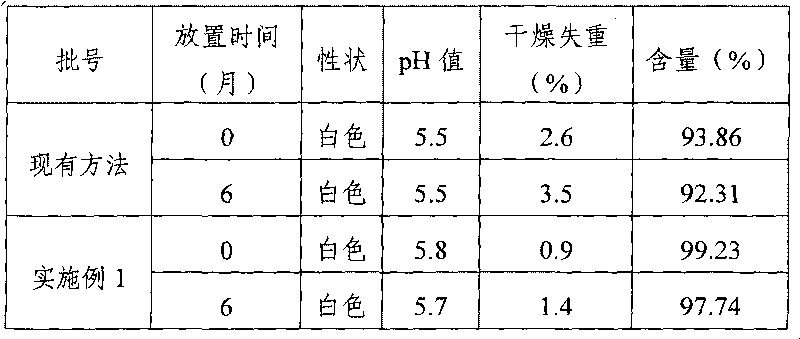
Examples
Embodiment 1
[0027] Gonadorelin 1.00g
[0028] Mannitol 200g
[0029] Phosphate buffered saline 30g
[0030] Add water for injection to 10L
[0031] (1) Preparation of medicinal solution: Weigh 200g of mannitol and add it to 5L of water for injection, stir until completely dissolved to obtain a mannitol solution; weigh 30g of phosphate buffered saline (wherein 26g of sodium dihydrogen phosphate and 4g of disodium hydrogen phosphate) are dissolved in Add 1L water for injection, then add to the above mannitol solution, mix well; add 1.0g / L activated carbon according to the full volume, boil for 15min, filter to remove carbon, cool to room temperature, add 1.00g gonadorelin, after completely dissolving, Add water for injection to 10 L, measure its pH to 5.8, filter through a 0.22 μm microporous membrane filter to a storage tank, prepare a medicinal solution, and set aside.
[0032] (2) Freeze-drying: Pre-lower the temperature of the condenser of the freeze-dryer (FCM50D type) to below -40°...
Embodiment 2
[0039] Gonadorelin 0.25g
[0040] Mannitol 800g
[0041] Phosphate buffered saline 30g
[0042] Add water for injection to 10L
[0043](1) Preparation of medicinal solution: Weigh 800g of mannitol and add it to 6L of water for injection, stir until completely dissolved to obtain a mannitol solution; weigh 30g of phosphate buffered saline (including 25g of sodium dihydrogen phosphate and 5g of disodium hydrogen phosphate) in Add 1L of water for injection, then add to the above-mentioned mannitol solution, mix well; add 2.0g / L activated carbon according to the full volume, boil for 30min, filter to remove charcoal, cool to room temperature, add 0.25g of gonadorelin, after completely dissolving, Add water for injection to 10 L, measure its pH to 5.8, filter through a 0.22 μm microporous membrane filter to a storage tank, prepare a medicinal solution, and set aside.
[0044] (2) Freeze-drying: Pre-lower the temperature of the condenser of the freeze-dryer (FCM50D type) to below...
Embodiment 3
[0050] Gonadorelin 1.00g
[0051] Mannitol 400g
[0052] Phosphate buffered saline 30g
[0053] Add water for injection to 10L
[0054] (1) Preparation of medicinal solution: Weigh 400g of mannitol and add it to 5L of water for injection, stir until completely dissolved to obtain a mannitol solution; weigh 30g of phosphate buffered saline (wherein 25g of sodium dihydrogen phosphate and 5g of disodium hydrogen phosphate) are dissolved in 1L of water for injection, then added to the above-mentioned mannitol solution, and mixed evenly; added activated carbon with a volume of 2.0g / L in full volume, boiled for 20min, filtered to remove charcoal, cooled to room temperature, added 1.00g of gonadorelin, after completely dissolving, supplemented Add water for injection to 10L, measure its pH value to 5.9, filter through a 0.22μm microporous membrane filter to a storage tank, prepare a medicinal solution, and set aside.
[0055] (2) Freeze-drying: Pre-lower the temperature of the con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com