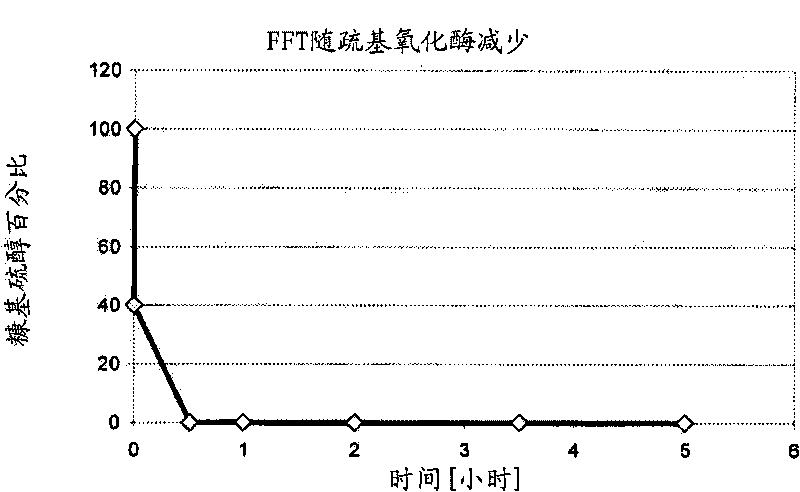
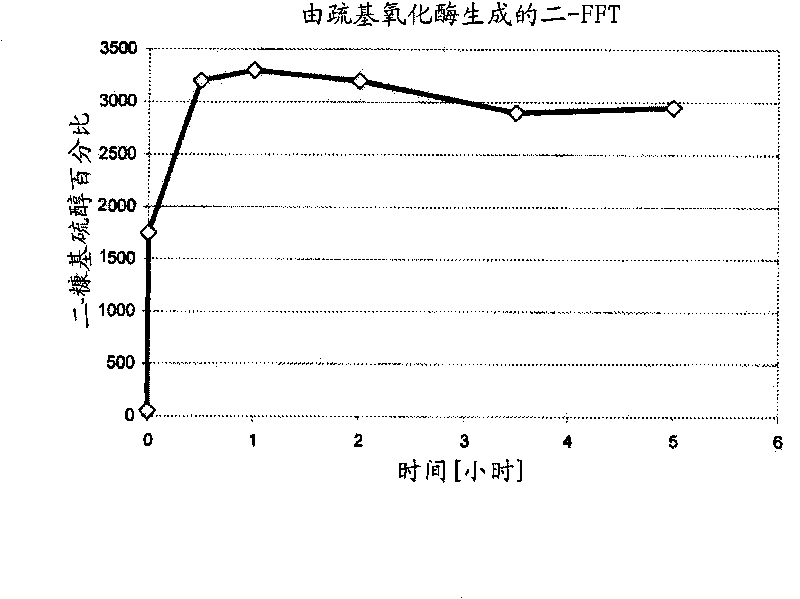
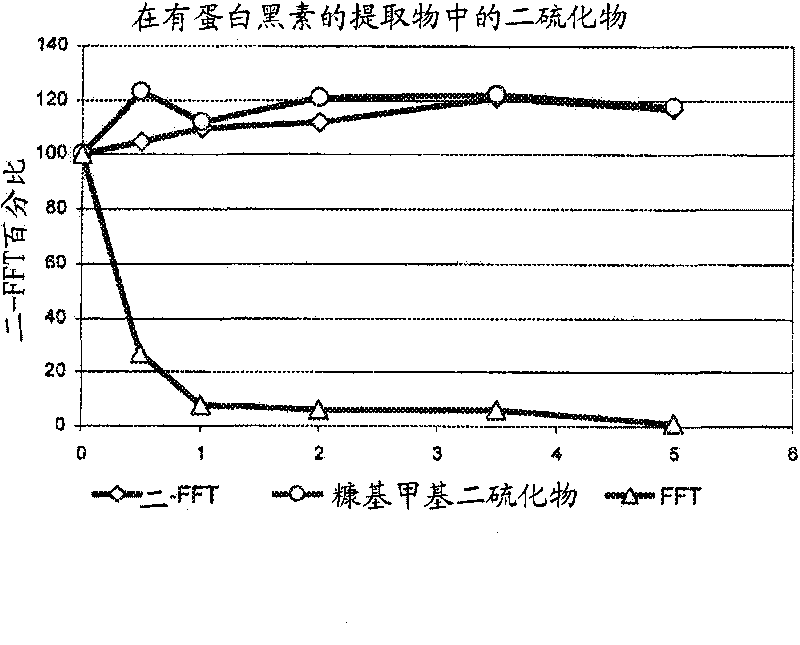
Increased stability of flavor compounds
A technology of flavoring agents and compounds, which is applied in the field of improving the processing of mercaptan-containing flavoring agents and mercaptans, and can solve problems such as instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Preparation of aromatic extract (1)
[0053] Ground roast coffee having a particle size of up to about 1.8 mm that has been moistened to a water content of about 50% by weight (relative to the ground dry roast coffee), in a percolator with saturated steam at a pressure of about 0.5 bar and about 100 °C for about 5 minutes. The steam laden with coffee ingredients condenses at a temperature of about 5° C. in an amount of condensate of about 5% by weight relative to the amount of dry roast coffee used.
[0054] melanin separation
[0055] Melanoproteins were isolated from cofiee brew by ultrafiltration using the following procedure: (a) Coffee beverage was separated into different fractions by ultrafiltration using a molecular weight cut off of 30 kDa ; (b) the residue (>30 kDa), the isolated melanin, was lyophilized and used for the reaction with FFT; (c) the filtrate (<30 kDa), the caffeine compound, was discarded.
[0056] Preparation of sulfhydryl oxidase solu...
Embodiment 2
[0071] Preparation of sulfhydryl oxidase solution (5)
[0072] 100 mg of sulfhydryl oxidase Ervlp from yeast (X-Zyme GmbH, Merowingerplatz 1A, 40225 Dusseldorf, Germany) was dissolved in 2 mL of Mcllvaine buffer (1 mM) with pH 7.5.
[0073] Preparation of Flavor Stabilized Aroma Extract (6)
[0074] 5 mL of the aroma extract solution of Example 1 (1) was supplemented with 2 mL of sulfhydryl oxidase solution (5) and stirred at 750 rpm at 40° C. for 2.5 hours in a 20 mL vial. Oxygen was injected into the vial every 30 minutes.
[0075] Evaluation by gas chromatography
[0076] The mixture of (1) and (5) was sampled by liquid-liquid extraction with dichloromethane followed by centrifugation. Aliquots of 1 μL were analyzed by GC-FID (HP 5890 series II) and GC-MS (Agilent 5973) by using DB 1701 capillaries (Agilent; 30 m x 0.32 mm ID x 1 μm FD). The GC oven was ramped from 40°C to 240°C at a heating rate of 5°C / min. A Gerstel CIS 3 syringe was used. A net increase in di...
Embodiment 3
[0082] Preparation of sulfhydryl oxidase solution (9)
[0083] 50 mg of sulfhydryl oxidase Ervlp from yeast (X-Zyme GmbH, Merowingerplatz 1A, 40225 Dusseldorf, Germany) was dissolved in 100 mL of Mcllvaine buffer (1 mM) with pH 7.54.
[0084] Preparation of aromatic extract (10) by enzymatic reaction
[0085] 60 mL of sulfhydryl oxidase solution (9) was supplemented in 150 mL of the aromatic extract solution (1) of Example 1, oxygen sparged every 30 minutes of reaction time, and incubated at 40° C. for 5 hours.
[0086] Evaluation of Shelf Life Stability
[0087] Reference sample 1
[0088] 5% of aroma extract (8) in dark Robusta coffee base has been diluted 1 / 5 in water.
[0089] sample 2
[0090] 5% of the enzyme-reacted aroma extract (10) in dark robusta coffee base has been diluted 1 / 5 in water.
[0091] Reference sample 1 and sample 2 were stored at 50°C for 8 days. Sensory evaluation of both samples was carried out by an expert taste panel by sniffing at vari...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com