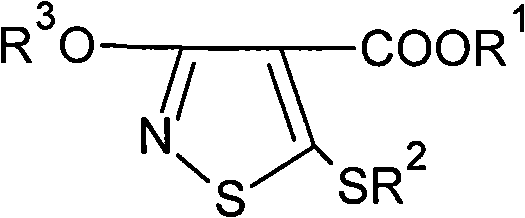
Preparation method of 4-carboxy-3-hydroxy-5-sulfydryl-isoniazthiolane
A technology of isothiazole salt and hydroxyl, applied in the field of preparation of pharmaceutical intermediates, can solve the problems of increased production cycle and cost, fewer manufacturers, poor product quality, etc., achieve significant economic benefits, good product quality, and reduce production cycle Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1), the synthesis of dicyano-U-vinyl mercaptide: add mass concentration 95% ethanol 500kg, 52kg industrial sodium hydroxide in 1000L glass-lined reactor, stir and dissolve clarification, cool to 0 ℃-10 with salt water °C, slowly add 40 kg of malononitrile, and stir for 30 minutes to obtain a light green transparent liquid. Control the temperature at 10-15°C, add 46 kg of carbon disulfide dropwise, and a large amount of light yellow solid precipitates. The temperature was raised to 20° C., and the reaction was carried out for 2 hours to obtain a yellow-white viscous substance, which was filtered and dried to obtain a light yellow solid. Dissolve the above-mentioned light yellow solid in 120kg of purified water, add 600kg of ethanol dropwise to the water after dissolving, and after the addition, lower the temperature to 0-5°C and grow the crystal for 2 hours. Filtrate, wash with absolute ethanol, and spin dry to obtain a light yellow solid, which is vacuum-dried to obta...
Embodiment 2
[0029] (1), the synthesis of dicyano-U-vinyl mercaptide: with embodiment 1.
[0030] (2), the synthesis of 4-carboxy-3-hydroxyl-5-mercapto-isothiazolium salt: add water 300kg in 1000L enamel reaction kettle, dicyano-U-vinyl mercaptide 92kg (dry), after stirring and dissolving , add 82 kg of hydrogen peroxide with a mass concentration of 30% dropwise. After adding, heat up to 20° C., stir for 1 hour, add 110 kg of sodium hydroxide, stir for 1 hour, transfer to a 500L stainless steel belt reflux reactor, and steam to reflux. For hydrolysis reaction, the reflux temperature is 115°C, reflux for 4 hours. After the reaction is completed, transfer the material to a 500L enamel kettle to cool down, add 300kg of water, control the temperature at 5-10°C, and add isooctanoic acid dropwise to adjust the pH value to 7.8-8.2. Cool down to about 0°C, grow crystals for 2 hours, filter, spin dry, wash with acetone-water slurry (volume ratio of acetone-water: water:acetone=1:1), spin dry, vacuu...
Embodiment 3
[0032] (1), the synthesis of dicyano-U-vinyl mercaptide: add mass concentration 95% ethanol 500kg, 73kg industrial potassium hydroxide in 1000L glass-lined reactor, stir and dissolve clarification, cool to 0 ℃-10 with salt water °C, slowly add 40 kg of malononitrile, and stir for 30 minutes to obtain a light green transparent liquid. Control the temperature at 10-15°C, add 46 kg of carbon disulfide dropwise, and a large amount of light yellow solid precipitates. The temperature was raised to 20° C., and the reaction was carried out for 2 hours to obtain a yellow-white viscous substance, which was filtered and dried to obtain a light yellow solid. Dissolve the above-mentioned light yellow solid in 120kg of purified water, add 600kg of ethanol dropwise to the water after dissolving, and after the addition, lower the temperature to 0-5°C and grow the crystal for 2 hours. Filtrate, wash with absolute ethanol, and spin dry to obtain a light yellow solid, which is vacuum-dried to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com