Production of bivalve tetraploid molluscs from diploid parents
A mollusk, tetraploid technology, applied in animal cells, climate change adaptation, animal husbandry, etc., can solve problems such as heavy weight, low competition of tetraploid larvae, and difficulty in genetic improvement.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1: Induction of polyploidy and reproduction of larvae
[0045] The diploid parents used for the experiment came from the natural wild-type diploid oyster spawning area in the French oyster Oléron Bay (Marennes Oléron Bay). Six female parents and four male parents were used for clustering and pairing through gonadal ablation. 15 million oocytes and 3 billion swimming sperm obtained from 10 parents were used for hybridization in 1 liter of filtered seawater at 25°C. The retention of the first polar body was affected by using mitostatin B (CB) dissolved in DMSO until the final concentration was 0.5 mg / L. Starting from the 10th minute after fertilization, CB was used for 15 minutes.
[0046] The protocol for inducing polyploidy is similar to the protocols for producing triploids that have been published in the literature (GUO et al., Biol. Bull., 183, 381-93, 1992; et al., Aquaculture, 174, 229-421999). However, the final concentration of CB was reduced (0.5 mg / L ins...
Embodiment 2
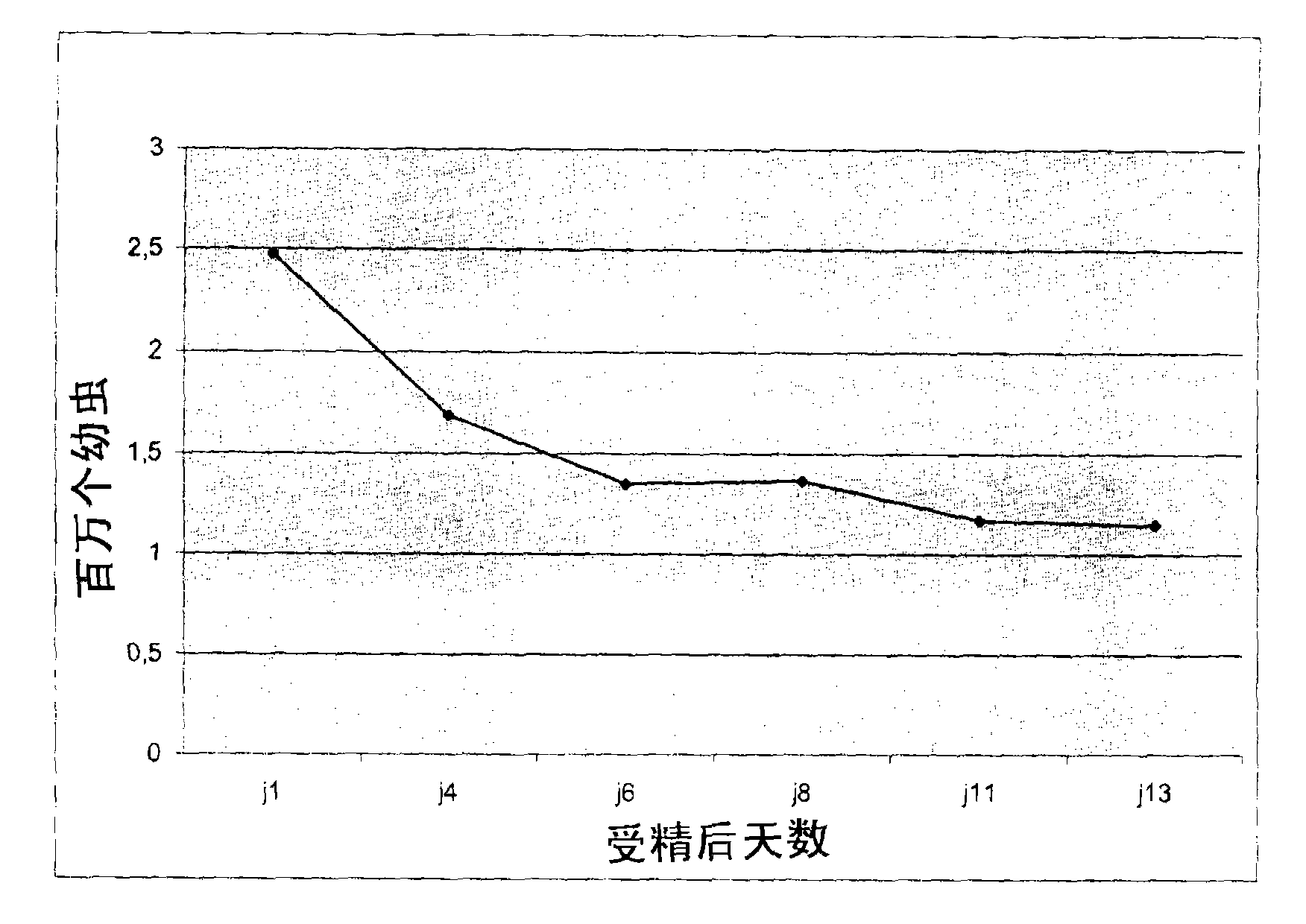
[0089] Example 2: Setting and growth of tetraploid oyster eggs
[0090] 1 month after placement
[0091] One month after placement, flow cytometry was used to check the presence of tetraploid oyster eggs in the successfully denatured and placed larvae population. Due to the small size of the oyster eggs at this stage, the operation was performed destructively on a sample containing some oyster eggs at some point. Put the oyster eggs into a 1.5 mL test tube. Then they were incubated in 1 mL of lysis buffer, and they were ground by using plastic stoppers. Finally, the released cell nuclei were filtered through a 30 μm mesh nylon filter (PartecCelltrics), and collected in a cell counting analysis test tube, labeled with DAPI and analyzed according to the method described in Example 1 above.
[0092] The cell count results allow us to confirm that in addition to the triploid and diploid populations, the population of tetraploid oyster eggs (29%) is also significantly present ( Figur...
Embodiment 3
[0115] Example 3: Production of tetraploid in mussels
[0116] By using the methods detailed in Examples 1 and 2, tetraploidies were effectively obtained in mussels (M. edulis and Mytilus galloprovincialis).
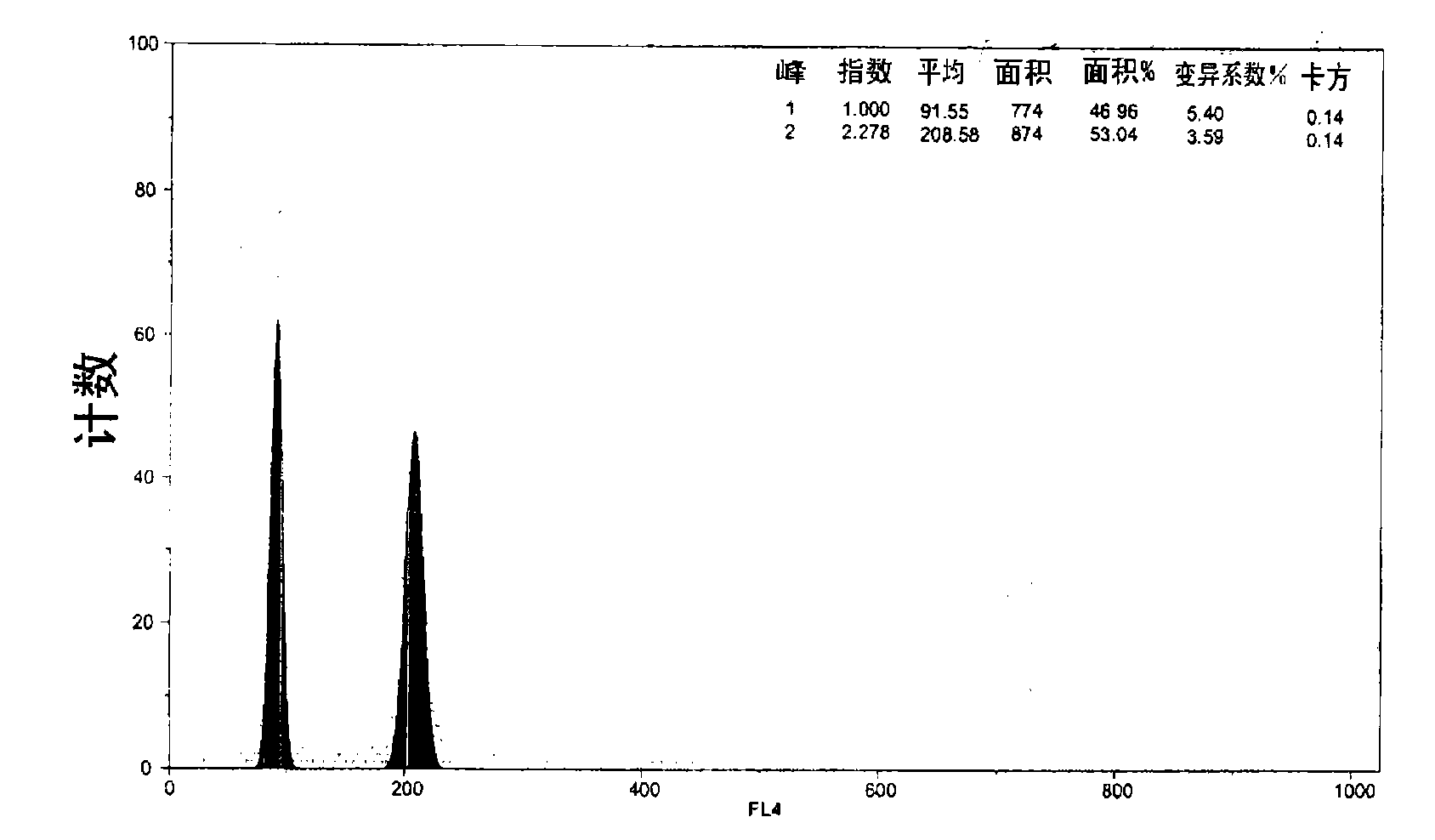
[0117] Among the mussels, the cell count results confirmed that in addition to the triploid and diploid populations, there is a predominant population of tetraploid oyster eggs. therefore,
[0118] -Control hybridization can provide an exclusive population of diploid nuclei ( Figure 37 , Peak "RN1");
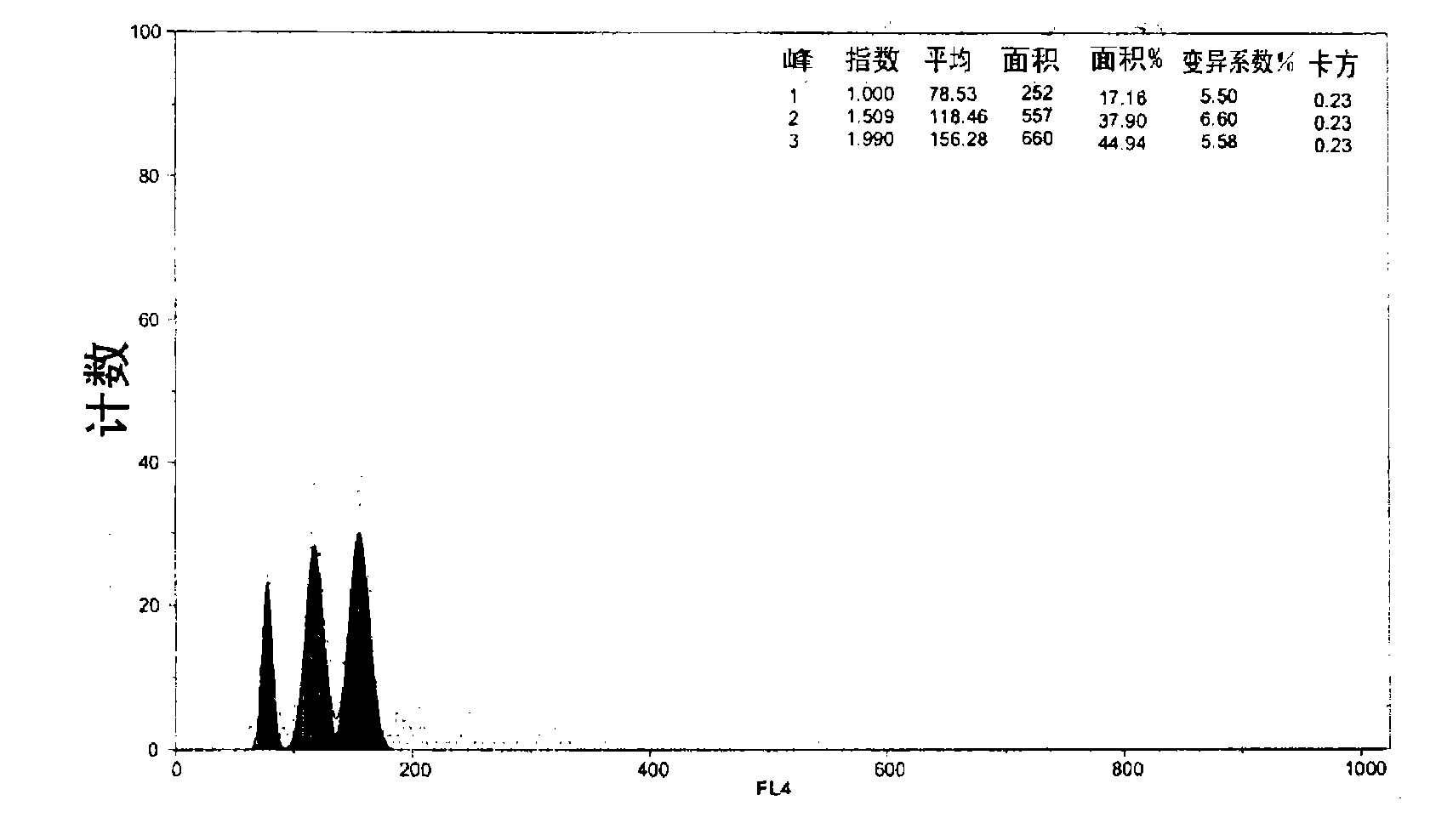
[0119] -The induced samples maintained by GPI showed three types of nuclear populations ( Figure 38 ), corresponding to the following ploidy levels: diploid (about 10% on average, peak "RN1"), triploid (about 20% on average, peak "RN2") and tetraploid (about 70% on average, Peak "RN3"). The resulting tetraploid individual is in the process of reproduction.
[0120] All in all, by using the method detailed in the above examples, in the same manner as that used for Pacific oyste...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com