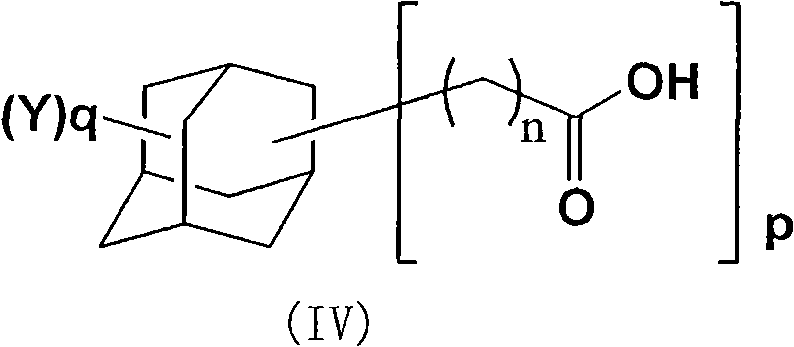
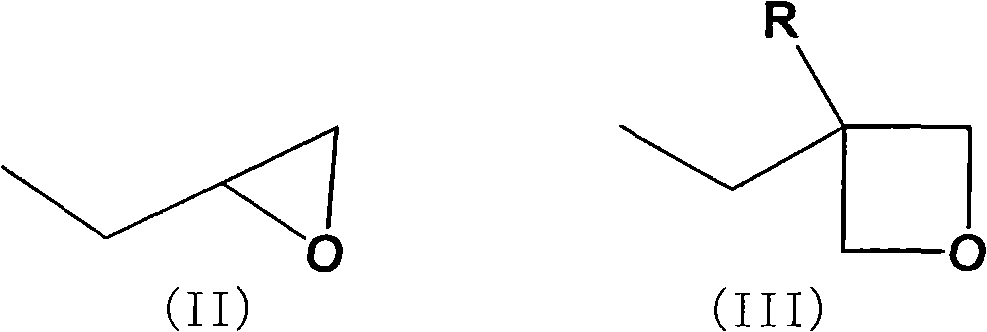Adamantane derivative, method for producing the same, resin composition containing the adamantane derivative and use thereof
A technology of resin composition and adamantane, which is applied in the photolithographic process of the patterned surface, instruments, electrical components, etc., can solve the problems of low yield, low reactivity, complex reaction path and other problems of cyclic ether compounds, and achieve the goal of sticking Good contact effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] [Synthesis of 1,3,5-adamantanetricarboxylic acid triglycidyl ester]
[0116] Add 1,3,5-adamantanetricarboxylic acid 30g (0.11mol), epichlorohydrin 124g ( 1.30 mol), 3 g of tetraethylammonium bromide, 90 g of toluene, and 40 g of 50 mass % sodium hydroxide aqueous solution were placed in a 130° C. oil bath for 5 hours and heated to reflux. At this time, a small amount of nitrogen gas was flowed and the mixture was stirred to allow the reaction to proceed while removing water flowing out as the reaction proceeded to the outside of the reaction system. Thereafter, the reaction liquid was cooled to room temperature, and the aqueous layer was washed with water until it became neutral, and then the solvent was distilled off from the organic layer to obtain a yellow viscous liquid.
[0117] Then, this yellow viscous liquid was dissolved in 175 g of toluene, 10.5 g of a 25% by mass aqueous sodium hydroxide solution was added, stirred in a 130° C. oil bath for 2 hours, and heat...
Embodiment 2
[0124] [Synthesis of 1,3-adamantane diacetate diglycidyl ester]
[0125] Add 50 g (0.20 mol) of 1,3-adamantane diacetic acid and 75 g (0.78 mol) of epichlorohydrin into a 500 mL round bottom flask equipped with a Dean-Stark reflux condenser, a stirrer, a thermometer, and a nitrogen inlet tube , 5 g of tetraethylammonium bromide, 225 g of toluene, and 48 g of a 50% by mass sodium hydroxide aqueous solution were placed in a 130° C. oil bath and immersed in a 130° C. oil bath for 3 hours and heated to reflux. At this time, a small amount of nitrogen gas was flowed and the mixture was stirred to allow the reaction to proceed while removing water flowing out as the reaction proceeded to the outside of the reaction system. Thereafter, the reaction liquid was cooled to room temperature, and the aqueous layer was washed with water until it became neutral, and then the solvent was distilled off from the organic layer to obtain a yellow viscous liquid.
[0126] Then, this yellow viscou...
Embodiment 3
[0143] 5 g of 1,3,5-adamantanetricarboxylic acid triglycidyl ester obtained in Example 1, 4.87 g of methyl hexahydrophthalic anhydride (manufactured by Nippon Chemical Co., Ltd., MH700) as an acid anhydride, 0.1 g of 1,8-diazabicyclo[5.4.0]undecene-7 octanoate (manufactured by San-Apro, SA102) as a curing accelerator, 2,6-di-tert-butyl as an antioxidant - 0.03 g of 4-methylphenol (BHT) was mixed at room temperature, defoamed, heated at 110° C. for 2 hours, and then heated at 150° C. for 3 hours to produce a cured product (a sheet with a film thickness of 3 mm).
[0144] Measure the glass transition temperature and light transmittance of the cured product of the resin composition according to the above-mentioned measurement methods (1) and (2), and carry out the light resistance test and long-term heat resistance test according to the above-mentioned evaluation tests (3) and (4). test. The evaluation results are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com