Alpha-glycosidase inhibitor compound in silkworm excrement total alkaloid and application thereof
A technology of glycosidase inhibition and total alkaloids, applied in the field of biomedicine, can solve problems such as heavy economic burden, lower quality of life, and great harm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Extraction of silkworm excrement total alkaloids
[0026] Preparation of silkworm excrement total alkaloid extract: take 1.2kg silkworm excrement, add 6L of deionized water, adjust pH to 3.0, keep warm at 40-50°C for 3 hours, and filter. The filter residue was extracted twice in the same way, and the three filtrates were combined, concentrated under reduced pressure to about 2.4 L, added with 3 L of absolute ethanol, mixed well, and left standing overnight. Centrifuge, take the supernatant and pass it through 001×7 type cation exchange resin, wash the non-adsorbed impurities with 50% (v / v) ethanol and deionized water successively, use 0.5-1.0mol / L ammonia solution to elute, collect α - For the positive part of the glycosidase inhibitory activity test, ammonia water was evaporated under reduced pressure, then decolorized by adsorption, eluted with absolute ethanol, and evaporated to dryness under reduced pressure to obtain 3.2 g of silkworm excrement total ...
Embodiment 2
[0027] Example 2: Separation and purification of active substances inhibiting α-glucosidase in silkworm excrement total alkaloid extract
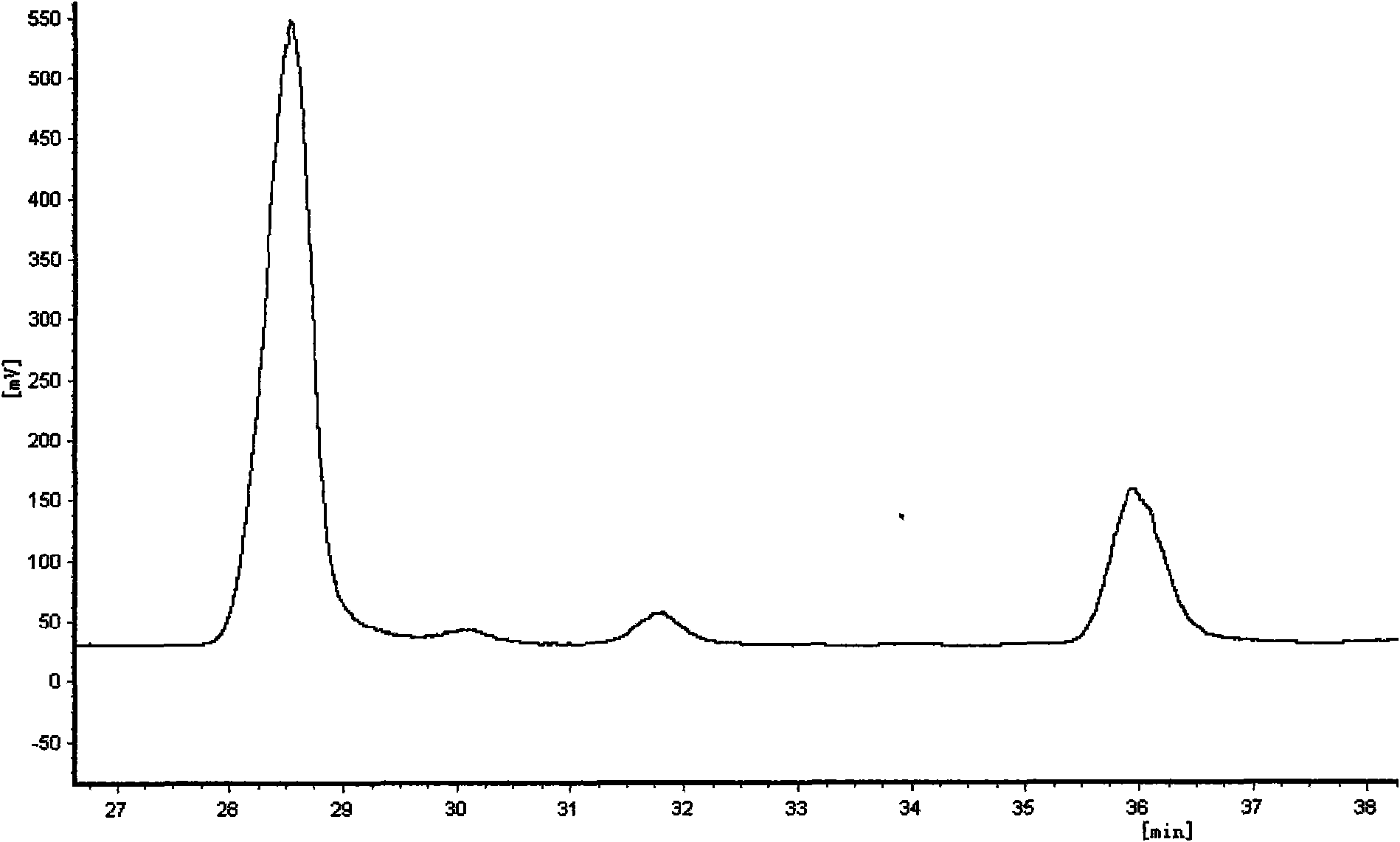
[0028] The silkworm excrement total alkaloid extract obtained in Example 1 was separated and purified by HPLC, and the chromatographic conditions were: cation exchange detection column: Hypersil SCX (250mm × 4.0mm, 5 μm); mobile phase was acetonitrile: water (90: 10v / v-60:40v / v); flow rate is 0.6mL / min; -2.0mL / min; evaporative light scattering detection; column temperature 40°C; injection volume 50-100μL; The material corresponding to the chromatographic peak of 35.9 minutes is evaporated to dryness, and the separated and purified sample is 1-deoxynojirimycin (DNJ); the material corresponding to the chromatographic peak with a retention time of 35.9 minutes is collected for multiple experiments, and evaporated to dryness The mobile phase obtained from the separated and purified sample is (2R,4R)-dihydroxy-5(R)hydroxymethylpiperidine.
Embodiment 3
[0029] Embodiment 3: the structure of (2R, 4R)-dihydroxy-5 (R) hydroxymethyl piperidine, 1-deoxynojirimycin (DNJ)
[0030] (2R,4R)-dihydroxy-5(R)hydroxymethylpiperidine:
[0031] (c=6.02×10 -5 , H 2 O microtube assay); 1 H-NMR (D 2 O, 300MHz) δ: 1.53 (1H, ddt, J=14.0, 11.4, 4.5Hz, H-3ax), 2.02 (1H, dddd, J=14.0, 5.0, 2.4Hz, H-3eq), 2.91 (1H, m, H-1ax), 2.98(1H, m, H-2), 3.24(1H, ddd, J=13.1, 4.5, 2.4Hz, H-1eq), 3.28(1H, t, J=10.4Hz, H -5), 3.54 (1H, ddd, J=11.4, 9.1, 4.8Hz, H-4), 3.74 (2H, m, H-6). 13 C-NMR (D 2(2, 300 MHz) δ: 28.7 (C-3), 42.0 (C-1), 57.8 (C-6), 60.2 (C-2), 69.7 (C-5), 7.06 (C-4).
[0032] 1-Deoxynojirimycin (DNJ):
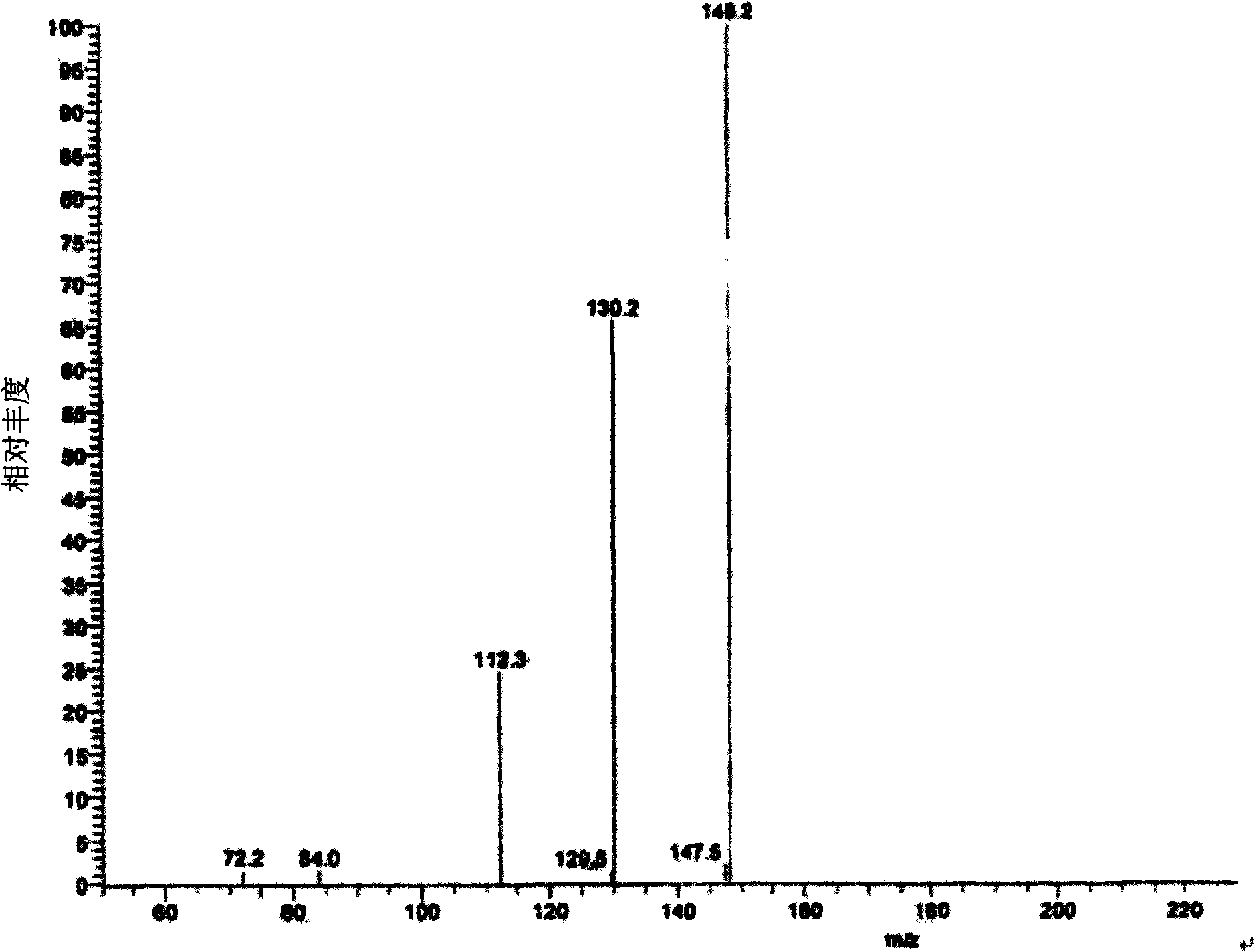
[0033] ESI-MS m / z: 164[M+H]+; 1H-NMR (D2O, 300MHz) δ: 3.86-3.63 (3H, m, 2H-6; H-3), 3.52-3.36 (3H, m, H-2; H-4; H-1e), 3.09 (1H, dd, H-1a), 2.86 (1H, t, H-5); 13C-NMR (D2O, 300MHz) δ: 76.2 (C-3 ), 67.7 (C-4), 66.9 (C-2), 59.9 (C-6), 57.7 (C-5), 45.8 (C-1). The above data are consistent with 1-deoxynojirimycin (DNJ) standard substance ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com