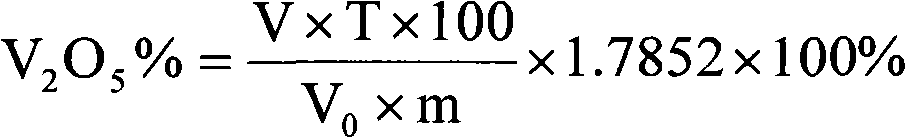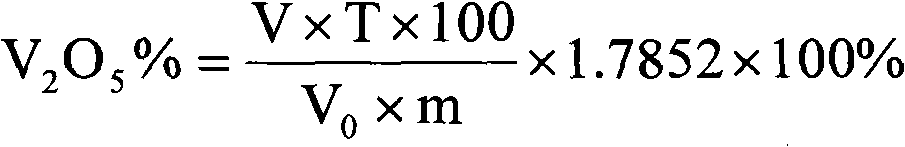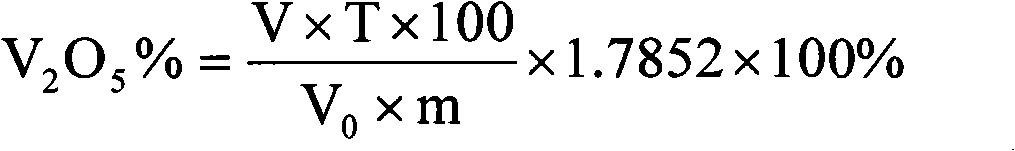Method for quickly determining vanadium in coal mine containing scherbinaite
A technology for rapid determination and stone coal mining, which is applied in chemical analysis by titration, preparation of test samples, and analysis using chemical indicators. It can solve problems such as time-consuming, difficult to decompose, and affect color judgment, and achieve color The effect of clear judgment, large sampling volume and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 will be taken from the stone coal mine (recorded as 1#) of a certain place in Hunan to carry out the mensuration of vanadium:
[0022] (1) Ore sample pretreatment and ore sample dissolution: the stone coal ore was crushed and finely ground until its particle size passed through a 200-mesh sieve, and then dried in an oven at 105°C for 2 hours. Take 5g of the dried ore sample in a plastic cup, gradually add 2mL of analytically pure hydrofluoric acid, and after leaching for 1 hour at room temperature, move all the ore pulp into a beaker, add 4mL of sulfuric acid (1+1), and leaching for 3 hours at 95°C. , filtered and washed while hot, and the volume of the filtrate was constant to 100mL;
[0023] (2) Drugs and reagents:
[0024] Sulfuric acid (analytical pure);
[0025] Sulfuric acid (1+1): prepared by mixing 1 volume of sulfuric acid with 1 volume of water;
[0026] Sulfuric acid (5+95): prepared by mixing 5 volumes of sulfuric acid with 95 volumes of wate...
Embodiment 2
[0046] Embodiment 2 (the preparation of medicine and reagent is the same as embodiment 1)
[0047] The stone coal mine (denoted as 2#) taken from a certain place in Guizhou was crushed and finely ground until its particle size passed through a 200-mesh sieve. It was dried in an oven at 105°C for 3 hours, and 10 g of the ore sample was taken in a plastic cup. Gradually add 4mL of analytically pure hydrofluoric acid, stir and leach for 2h at room temperature, add 5mL of sulfuric acid (1+1), and leach for 4h at 95°C, filter and wash while hot, and make the volume of the filtrate to 100mL. Take 1mL of the filtrate into a 300mL Erlenmeyer flask, and rinse the inner wall of the bottle with a small amount of water. Add 20mL of sulfuric acid (1+1), 5mL of phosphoric acid (1+1), dilute with water to about 100mL, place to cool, add 5mL of ferrous sulfate, wait for 2min, add potassium permanganate dropwise under artificial shaking conditions until the solution turns red , wait for 5 min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com