Coal bed gas deoxidation catalyst as well as preparation method and application thereof
A deoxygenation catalyst, catalyst technology, applied in catalyst activation/preparation, chemical instruments and methods, metal/metal oxide/metal hydroxide catalyst, etc. problem, to achieve the effect of eliminating the active oscillation phenomenon, improving stability and stabilizing the combustion process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 393.574g Zr(NO 3 ) 4 ·3H 2 O was dispersed in 800ml of deionized water, heated to 75-80°C with stirring to dissolve it completely, and the solution was cooled to 1000ml to obtain Zr(NO 3 ) 4 solution. Take 100ml of the above solution, together with 43.465g Ce(NO 3 ) 3 ·6H 2 O, 37.876gAl(NO 3 ) 3 9H 2 O was dissolved in deionized water and fixed to 300ml. Under the condition of constant stirring, 25-28% ammonia water was dripped into the above mixed solution with a separatory funnel. The amount of ammonia water was controlled according to the pH value until the pH value reached 8-9. Then the obtained precipitate was fully stirred for 2 hours, suction filtered, and the filter cake was washed 3 times with 1200ml of deionized water, and the washed filter cake was placed in a vacuum oven at 60°C for 20 hours and dried in a muffle furnace at 2.5 The heating rate of °C / min was raised to 500 °C for 2 hours to obtain 34.314g weight percent composition of 50% CeO 2 -35...
Embodiment 2
[0056] Add 30L deionized water in the reactor of 70L, the urea of 6459g, the (NH 4 ) 2 Ce(NO 3 ) 6 into the kettle, and finally Zr(NO with a concentration of 1M 3 ) 4 2.39 L of the solution and an additional 12 L of deionized water were added to the kettle. Heat the feed liquid in the kettle until the urea decomposes and further heat to a boiling state (98-100°C) and stir for 4 hours, then stop heating and continue to stir and age for 2 hours to obtain CeO by homogeneous co-precipitation 2 -ZrO 2 Complex oxide precursors. Centrifugally filter the prepared precipitate, fully wash the filter cake twice in the reaction kettle with 60 L of boiling water under stirring, and perform centrifugal filtration after each washing. After washing and filtering twice with deionized water, the obtained filter cake was fully dispersed in 10 L of isopropanol solvent to take away the residual water in the precipitate, and the isopropanol was centrifuged and filtered. The resulting prec...
Embodiment 3
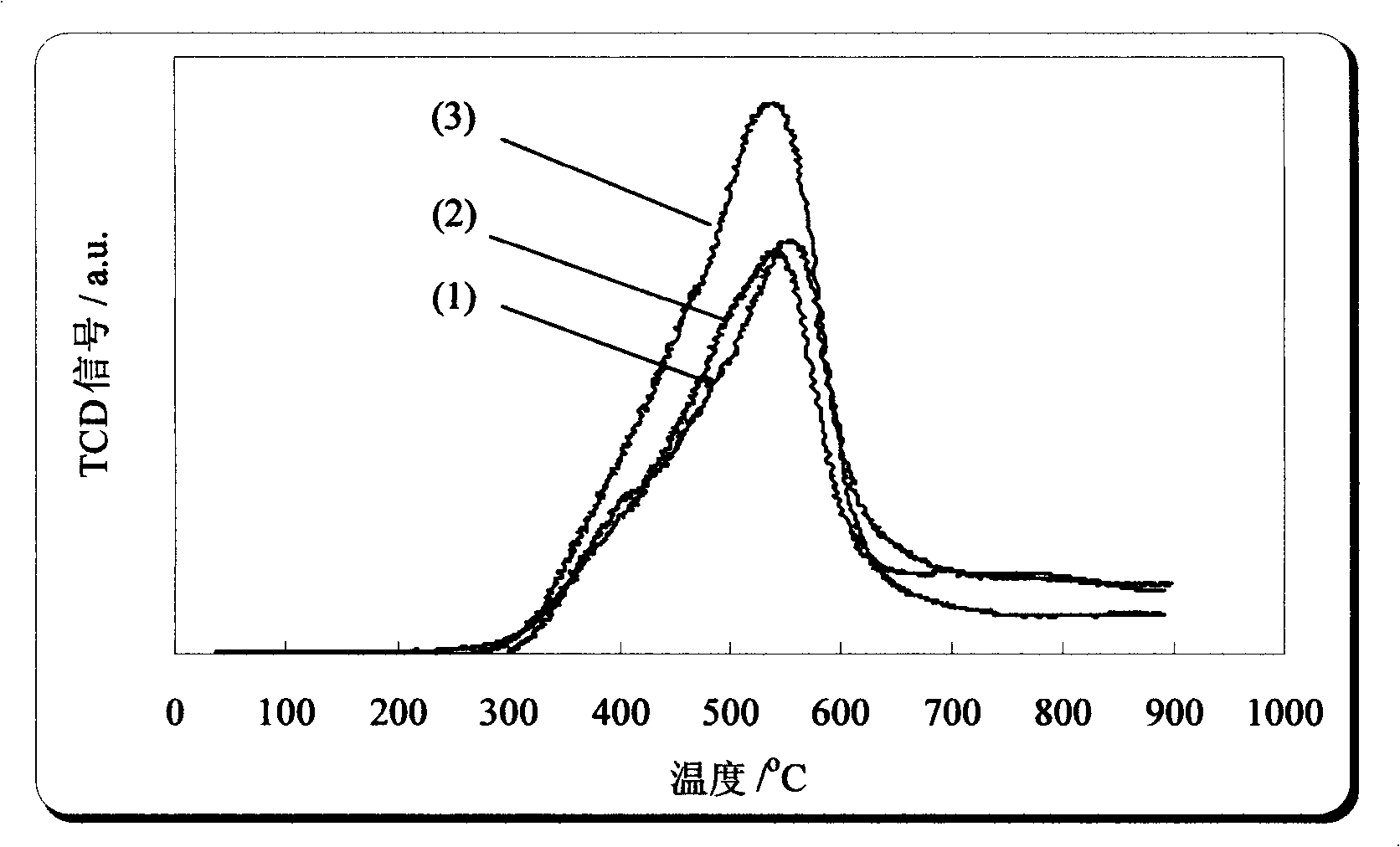
[0060] Adopting the method for preparing Ce-Zr composite oxide described in example 2 to prepare weight percentage composition is 58%CeO 2 -42% ZrO 2 Ce-Zr composite oxide powder and sampled for BET and H 2 - TPR characterization. The BET specific surface area of the Ce-Zr composite oxide is 120.4m 2 / g, its H 2 - The TPR spectrum is shown in figure 1 , the reduction process H 2 The consumption is 810.7μmol / g.
[0061] Replace the Ce-Zr-Al ternary compound or microcrystalline mixture in Example 1 and Example 2 with the Ce-Zr composite oxide powder prepared above, and prepare CeO in Example 1 and Example 2 2 Ce-Zr composite oxide slurry is prepared by the method of based composite oxide slurry, and the coating of Ce-Zr composite oxide, MgO and noble metal Pd is carried out with the same coating coating method, and the obtained specific composition is 0.18% The catalyst sample of Pd / 3.12%MgO / 12.76%Ce-Zr-Ox / 83.94% cordierite, the sample code is Example-3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com