Aeromonas hydrophila inactivated vaccine and preparation thereof
A technology of Aeromonas hydrophila and inactivated vaccine, which is applied in the field of Aeromonas hydrophila inactivated vaccine and its preparation, can solve the problems of poor safety, high cost, unstable immune effect and the like, and reduce mortality rate , the effect of reducing economic losses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Isolate the pathogenic bacteria Aeromonas hydrophila from sick fish suffering from bacterial septicemia:
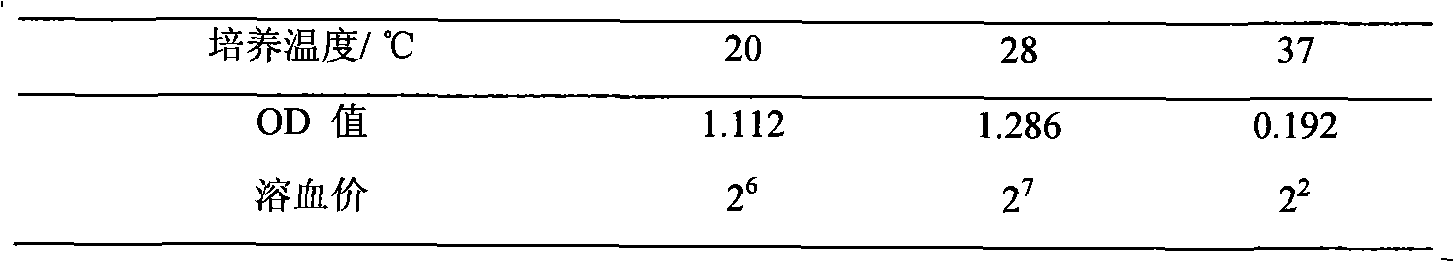
[0034] a. Aseptically take the liver, spleen and kidney of diseased or dying carp, inoculate them on normal nutrient agar medium by streaking, culture at 28°C for 24 hours, pick a single colony to obtain a pure culture, and transfer it to the normal nutrient agar slant Stored on culture medium for identification. Typical colonies on ordinary nutrient agar plates are round, colorless or light yellow, translucent, smooth, moist, with neat edges and no water-soluble pigments.
[0035] b. Take the pure cultured bacteria on the slant above, make a smear specimen, and check the bacterial morphology by Gram staining microscope. The results of ordinary light microscope examination are Gram-negative short bacilli, with blunt round or straight ends, most of which are arranged in single, and a few are arranged in two, without capsule and without spores. Then inoculated ...
Embodiment 2
[0072] This embodiment is basically the same as Embodiment 1, except that the liquid vaccine is made into a solid powder vaccine by a freeze dryer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com