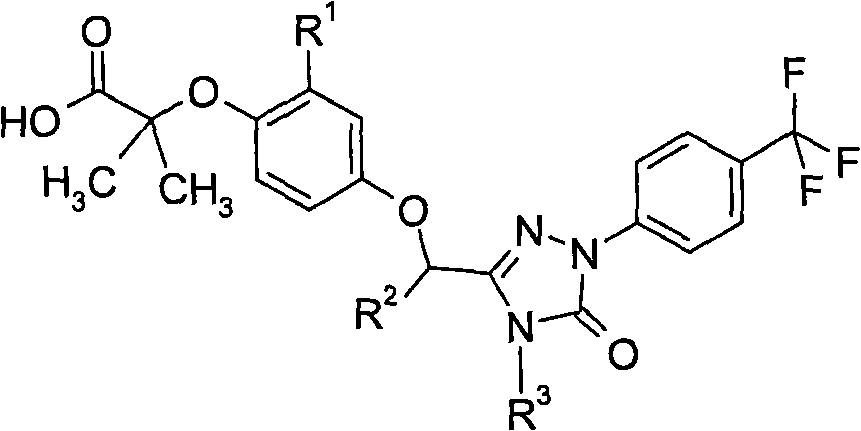
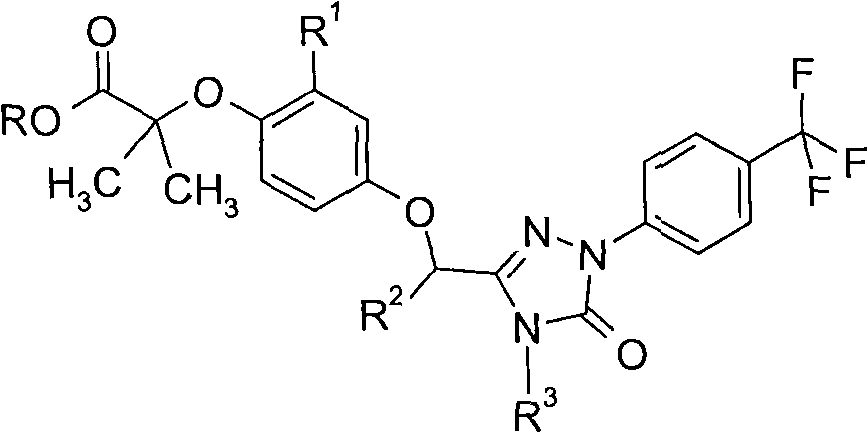
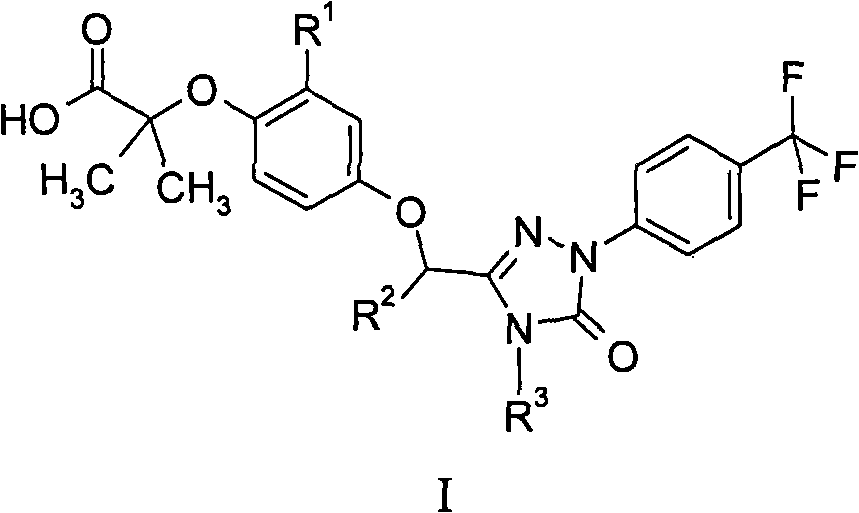
Peroxisome proliferator activated receptor modulators
A technology of compounds and intermediates, applied in the field of peroxide proliferator-activated receptor modulators, can solve the problems of drug treatment failure, adverse drug reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0079] Preparation via Alcohol Intermediate Route A
[0080] preparation 1
[0081] 2-Benzyloxy-N-isopropyl-acetamide
[0082]
[0083] To a solution of isopropylamine (10.7 mL, 125 mmol) in dichloromethane was added benzyloxyacetyl chloride (7.8 mL, 50 mmol) at 0°C. After stirring overnight at room temperature, it was concentrated and partitioned between ethyl acetate and 1N HCl. Dry the organic phase (Na 2 SO 4 ) and concentrated to give a white solid: 10.4 g. 1 H-NMR (CDCl 3 )δ7.36(m, 5H), 6.38(bs, 1H), 4.56(s, 2H), 4.11(m, 1H), 3.95(s, 2H), 1.73(d, 6H).
[0084] preparation 2
[0085] 2-Benzyloxy-N-isopropyl-thioacetamide
[0086]
[0087] Add Lawesson's reagent (12.1 g, 30 mmol) to a toluene (100 mL) suspension containing 2-benzyloxy-N-isopropyl-acetamide (10.4 g, 50 mmol), and stir under reflux The mixture was left overnight. evaporated to dryness in Et 2 The residue was suspended in O / hexane and filtered. The filtrate was concentrated and purifie...
Embodiment 1
[0134] 2-{4-[4-isopropyl-5-oxo-1-(4-trifluoromethyl-phenyl)-4,5-dihydro-1H-[1,2,4]triazole- 3-ylmethoxy]-2-methyl-phenoxy}-2-methyl-propionic acid
[0135]
[0136] To room temperature containing 2-{4-[4-isopropyl-5-oxo-1-(4-trifluoromethyl-phenyl)-4,5-dihydro-1H-[1,2,4 ]triazol-3-ylmethoxy]-2-methyl-phenoxy}-2-methyl-propionic acid ethyl ester (588mg, 1.12mmol) in dioxane (3ml) was added lithium hydroxide ( 1.69ml, 3.36mmol, 2.0M in water) and heated to 50°C overnight. The mixture was concentrated and in Et 2 Partition the residue between O and 1N HCl, wash the organic phase with water, dry (MgSO 4 ), filtered and concentrated to give the desired product 539mg. LC-MS: 494 (M+1).
[0137] Synthetic Method 2 (Scheme 2)
[0138] Formation of an ester-protected intermediate, N 4 deprotection of the position, followed by alkylation and base hydrolysis
[0139] Preparation 13
[0140]2-methyl-2-{2-methyl-4-[5-oxo-1-(4-trifluoromethyl-phenyl)-4,5-dihydro-1H-[1,2,4 ]...
Embodiment 2
[0148] 2-{4-[4-(2-methoxy-ethyl)-5-oxo-1-(4-trifluoromethyl-phenyl)-4,5-dihydro-1H-[1, 2,4]triazol-3-ylmethoxy]-2-methyl-phenoxy}-2-methyl-propionic acid
[0149]
[0150] At room temperature, 2-{4-[4-(2-methoxy-ethyl)-5-oxo-1-(4-trifluoromethyl-phenyl)-4,5-dihydro-1H -[1,2,4]triazol-3-ylmethoxy]-2-methyl-phenoxy}-2-methyl-propionic acid ethyl ester (479 mg, 0.889 mmol) was dissolved in 30 mL of THF and 1:1 mixture of ethanol. Add 2.2 mL of potassium hydroxide 2N aqueous solution, and stir at room temperature for 18 hours. Concentrate in vacuo and acidify to pH4. Dilute with ethyl acetate and separate the phases. The aqueous layer was extracted twice with ethyl acetate. The organics were combined and washed successively with brine and water, dried over magnesium sulfate, filtered and the solvent was evaporated to give 417 mg of the title compound. ES-MS: 510 (M+1).
[0151] In Table 1, each example was prepared essentially as described in Example 1 by preparing an al...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com